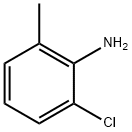
2-chloro-6-methyl aniline: uses and synthesis
Introduction
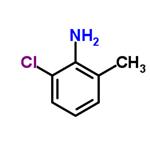
2-chloro-6-methylaniline is a Clear Colourless to Pale Purple oil. This compound is an important organic synthesis intermediate widely applied to chemical pharmacy, pesticides and organic synthesis.
Uses
In the aspects of chemical pharmacy and pesticides, 2-chloro-6-methylaniline can be used as a reaction starting material to participate in the preparation processes of tyrosine kinase inhibitors dasatinib, indazole derivatives with anti-breast cancer activity, 2-methyl-6-chlorophenyl sulfonylurea derivatives with bacteriostatic activity and the like; in the aspect of organic synthesis, 2-chloro-6-methylaniline can introduce chemical groups such as hydrazine, nitro, cyano, halogen (F, Cl, Br), and the like into an aromatic ring through diazotization reaction, thereby participating in wide chemical reactions.
Found in crude oil extract
Crude oil from the Shengli oilfield of China was extracted with N, N-dimethylformamide (DMF) for separation and enrichment of organic chlorides. The resulting extract with a relatively higher chlorine concentration was analyzed with gas chromatography-mass spectrometry (GC/MS). Among the identified heteroatom compounds in the extracted sample, nitrogen-containing compounds were most abundant in the extracted sample, followed by chlorine-, sulfur-, and oxygen-containing compounds, respectively. Four organochlorines with an aromatic core structure, i.e., 5-chloro-2-methylaniline, 2-chloro-6-methylaniline, 4-chloro-2-nitrotoluene, and 1-(5-chloro-2-hydroxyphenyl)ethanone, were identified from crude oil extract. The dominant abundant organic chloride is 5-chloro-2-methylaniline, with relative content of 65.1% in the total of identified organic chlorides, followed by 1-(5-chloro-2-hydroxyphenyl)ethanone of 16.3%, 2-chloro-6-methylaniline of 14.5%, and 4-chloro-2-nitrotoluene of 4.1%, respectively.
Synthesis
At present, the literature reports the preparation methods of 2-chloro-6-methylaniline, including the following methods:
(1) in patent CN110015963A, 4-amino-3-methylbenzenesulfonic acid and acetic anhydride are used as raw materials and sodium hydroxide is used as an acid-binding agent to prepare 4-acetamido-3-methylbenzenesulfonic acid sodium salt by a one-pot method. Then 2-chloro-6-methylaniline is obtained through chlorination, deamination protection and decarboxylation reaction.
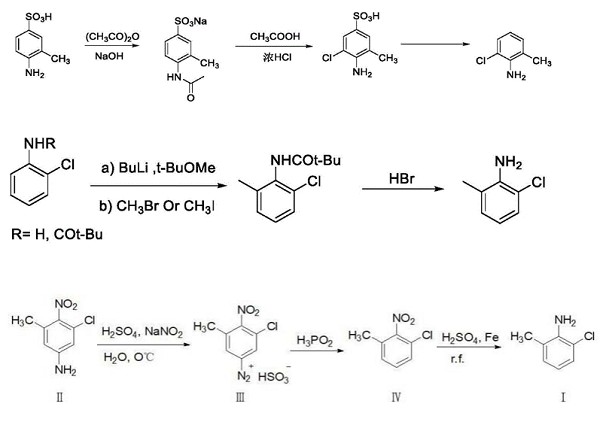
(2) Thomas A. and the like take 2-chloroaniline as a raw material. First, tert-butyl lithium and methyl tert-butyl ether are utilized to mediate the 2-chloroaniline to form a six-membered ring transition state. Then, a methylation reaction is carried out through methyl bromide. The transition state is dissociated under an acidic condition, and finally, the methyl aniline with a yield of 65.9% is obtained.
(3) Qu et al. discloses a preparation method of 2-chloro-6-methylaniline, which takes 3-chloro-5-methyl-4-nitroaniline as a starting material, water as a solvent and sulfuric acid as a reactant, eliminates amino groups through diazotization reaction, reduces by hypophosphorous acid to obtain an intermediate, and finally takes iron powder as reducing nitro groups to prepare the 2-chloro-6-methylaniline through a one-pot reaction. The method has the advantages of short reaction steps, mild reaction conditions, high product yield and low cost, and provides a general new method for preparing the 2-chloro-6-methylaniline.
References:
[1] XIAOHUI LI. Separation and Characterization of Organic Chlorides in a Chinese Crude Oil[J]. Bulletin of the Korean Chemical Society, 2018, 39 4: 411-590. DOI:10.1002/bkcs.11422.You may like
Lastest Price from 2-Chloro-6-methylaniline manufacturers
US $0.00-0.00/KG2025-04-04
- CAS:
- 87-63-8
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton
US $0.00-0.00/kg2025-02-20
- CAS:
- 87-63-8
- Min. Order:
- 0.1kg
- Purity:
- 0.99
- Supply Ability:
- 5 tons