Baloxavir Marboxil: A Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza
General Description
Baloxavir marboxil is an A Cap-Dependent Endonuclease Inhibitor medication used for the treatment of influenza. It has a unique mechanism of action that inhibits viral gene transcription. Some of its benefits include the fact that it can be administered as a single dose, making it convenient and cost-effective. It also shows efficacy against both influenza A and B viruses. However, there are still uncertainties surrounding its use in certain patient populations, the potential development of resistance, and limited data on its effectiveness and safety beyond the first 48 hours of symptoms. Further research is necessary to fully understand its efficacy and safety in different situations.

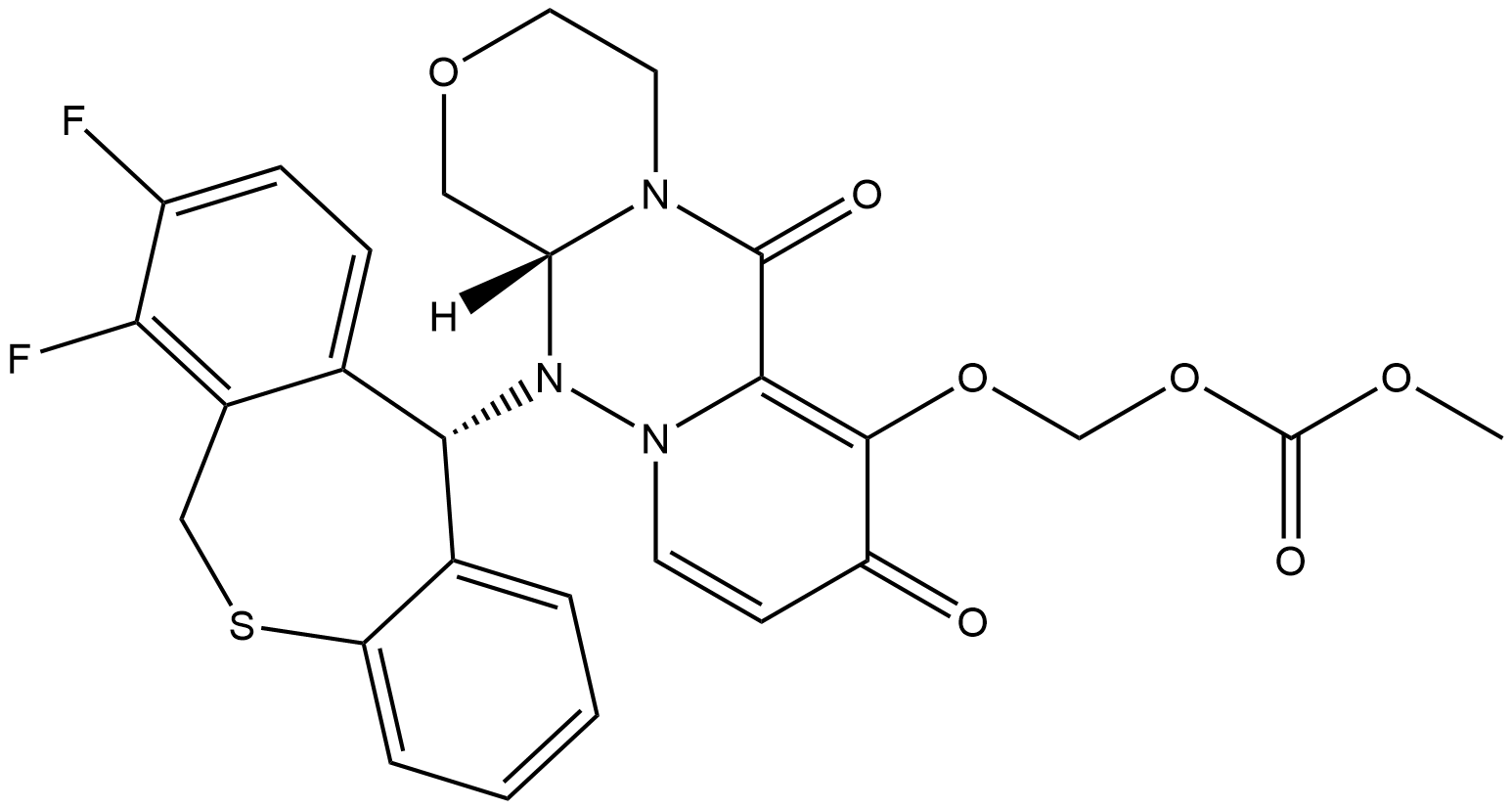
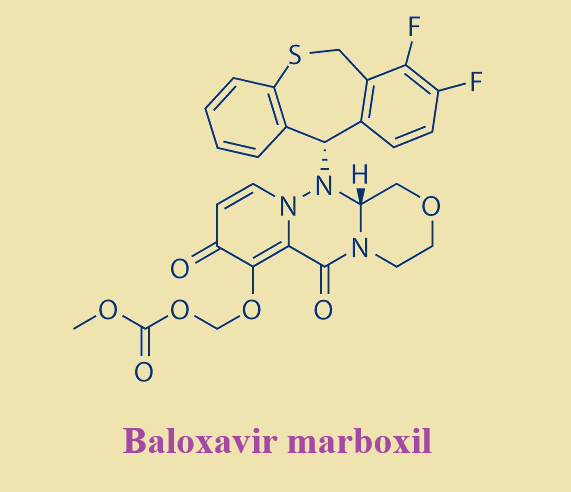
Figure 1. Baloxavir marboxil
Pharmacology
Baloxavir marboxil is a prodrug that is converted into the active drug, baloxavir acid. It targets the third stage of viral replication, which is different from other commonly used antiviral medications. Neuraminidase inhibitors and M2 ion-channel inhibitors work by inhibiting viral release and uncoating, respectively. Baloxavir acid, on the other hand, inhibits the endonuclease activity of the polymerase acidic (PA) protein, which is essential for viral gene transcription. By blocking this enzyme, baloxavir prevents the synthesis of viral mRNA. It has a long half-life of 79.1 hours, allowing for single-dose administration. Baloxavir is metabolized through UGT1A3 and CYP3A4, with UGT1A3 being the main contributor. Although there is a potential for drug interactions, no significant effects were observed with strong CYP3A and UGT inhibitors. However, medications containing calcium, aluminum, magnesium, or iron should not be coadministered due to chelation. Baloxavir is predominantly excreted through feces. Its pharmacokinetic profile appears similar in patients with moderate hepatic impairment but requires monitoring in patients with severe renal or hepatic impairment as they were not included in clinical trials.1
Clinical practice
Baloxavir marboxil is a medication used to treat influenza and offers several advantages for patients. Being administered as a single dose solves the issue of medication nonadherence and is particularly convenient during clinic visits. In comparison to peramivir, another single-dose medication, baloxavir marboxil is considerably more cost-effective. Patients also prefer oral medications over intravenous injections, making it a favorable option. Another benefit is its unique mechanism of action. It has shown high potency against various strains of influenza A and B viruses, including those that are resistant to other antiviral drugs like oseltamivir. This lack of cross-resistance makes it a valuable alternative for patients with resistant viruses. However, there are still uncertainties surrounding its use. Currently, there is no recommendation for strain and susceptibility testing before initiating treatment, so oseltamivir remains the preferred option in most cases. Additionally, there is limited data on the efficacy and safety of baloxavir marboxil beyond 48 hours of symptom onset, so off-label use in this context cannot be recommended. For critically ill patients, oseltamivir is the only agent with sufficient data to support its use. While baloxavir marboxil may be an option for hospitalized patients who do not respond to oseltamivir, there are no completed studies in this patient population. Combination therapy with baloxavir marboxil and oseltamivir has shown promise in animal studies, but more clinical data is needed to recommend this approach. It should be noted that baloxavir marboxil is not immune to resistance, as some influenza strains have been found to develop resistance, particularly with the I38T substitution. The clinical implications of this resistance are currently unclear. In conclusion, baloxavir marboxil offers advantages such as single-dose administration and a unique mechanism of action. However, uncertainties remain regarding its use in certain patient populations and the potential for resistance development. Further research and clinical trials are needed to establish its efficacy and safety in different contexts. 2
Reference
1. Xofluza™ [package insert]. South San Francisco, CA: Genen- tech USA, Inc; 2018.
2. Yang T. Baloxavir Marboxil: The First Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza. Ann Pharmacother. 2019;53(7):754-759.
Related articles And Qustion
Lastest Price from Baloxavir marboxil manufacturers

US $0.00/kg2025-06-20
- CAS:
- 1985606-14-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $10.00/ASSAYS2025-05-04
- CAS:
- 1985606-14-1
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton