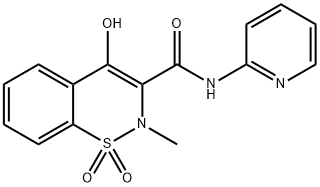
Piroxicam: Pharmacokinetics, Mechanism of Action and Toxicology Studies
General Description
Piroxicam is a nonsteroidal anti-inflammatory drug with linear pharmacokinetics and a half-life of 30 to 60 hours. It primarily inhibits prostaglandin synthesis, but recent studies suggest that its anti-inflammatory effects may also involve effects on inflammatory cell activation. Toxicology studies on rats have shown that high doses of piroxicam can cause advanced parturition, prolonged labor, reduced live births, and fetal mortality. Lactating females treated with piroxicam can experience toxicity and impaired lactation. While the usual human dose is well below the threshold dose for these effects, caution should be exercised in pregnant women taking piroxicam. Lower doses of piroxicam cause little toxicity and do not affect postnatal development.
Figure 1. Capsules of piroxicam
Pharmacokinetics
Piroxicam is a nonsteroidal anti-inflammatory drug that exhibits linear pharmacokinetics following administration of single doses. The maximum plasma concentrations are typically reached within 2 hours, although this may vary between 1 and 6 hours among different individuals. Steady-state concentrations, achieved after 7 to 12 days of repeated daily doses of 20mg, show peak plasma concentrations ranging from 4.5 to 7.2 mg/L and trough concentrations from 3.9 to 5.6 mg/L. When a loading dose of 40mg is administered for the first 2 days, approximately 75% of steady-state plasma concentrations are attained by the second day. However, without a loading dose, it may take up to 8 days to reach this level. Omitting a single day's dosage after 7 days of continuous medication has minimal impact on piroxicam plasma concentration, unlike other NSAIDs such as diclofenac, indomethacin, and flurbiprofen. Piroxicam penetrates into the synovial fluid of patients with rheumatoid arthritis, with concentrations approximately 40% of those in plasma. In breast milk, piroxicam concentrations are around 1% of maternal plasma levels. The drug is primarily eliminated through biotransformation, with metabolites showing little to no anti-inflammatory activity. The elimination half-life of piroxicam ranges from 30 to 60 hours in healthy individuals due to a low systemic clearance rate. Age and renal function have limited influence on piroxicam elimination, but plasma concentrations are increased in patients with severe liver insufficiency. Therapeutic effect is not closely associated with either plasma or joint fluid concentrations of piroxicam. 1
Mechanism of Action
Piroxicam is known to inhibit prostaglandin synthetase. This has been traditionally thought to be the mechanism of action for their anti-inflammatory effects in rheumatic disorders. However, recent studies suggest that the anti-inflammatory effects of NSAIDs may also be due to their effects on inflammatory cell activation. While piroxicam, ibuprofen, and indomethacin all inhibit prostaglandin synthetase, they exhibit different inhibitory effects on neutrophil functions depending on the stimulus used. This suggests that the effects on polymorphonuclear leucocyte function are not solely dependent on prostaglandin inhibition. Therefore, the mechanism of action of piroxicam is likely to involve more than just the inhibition of prostaglandins. It may also involve effects on inflammatory cell activation, although further research is needed to fully understand this mechanism. 2
Toxicology Studies
Toxicology studies have been conducted on piroxicam, confirming its effects on parturition in rats. In pregnant rats given piroxicam at doses of 5 and 10 mg/kg from day 15 to day 21 or 22 after insemination, parturition was advanced in some animals followed by death. Prolonged labour was observed in a dose and duration-dependent manner. The number of live pups per litter was also reduced, and fetal mortality increased with the length of gestation. Treatment for shorter periods did not affect parturition. Additionally, lactating females treated with piroxicam at 10 mg/kg experienced marked toxicity, with approximately one-third dying 7 to 13 days postpartum. Lactation was impaired, as evidenced by the lack of milk in the stomachs of their offspring. While the usual daily dose of piroxicam for humans is 20mg, equivalent to about 0.3 mg/kg, well below the threshold dose (2 mg/kg) at which effects were observed in rats, it is possible that gestation and labor could be prolonged in pregnant women taking piroxicam. Autopsy revealed gastric ulceration and hemorrhage, and peritonitis. Lower doses of piroxicam caused little toxicity and did not influence postnatal development. 3
Reference
1. Brogden RN, Heel RC, Speight TM, Avery GS. Piroxicam. A reappraisal of its pharmacology and therapeutic efficacy. Drugs. 1984;28(4):292-323.
2. Abramson S, Edelson H, Kaplan H, Given W, Weissmann G. The neutrophil in rheumatoid arthritis: its role and the inhibition of its activation by nonsteroidal antiinflammatory drugs. Semin Arthritis Rheum. 1983;13(1 Suppl 1):148-153.
3. Perraud J, Stadler J, Kessedjian MJ, Monro AM. Reproductive studies with the anti-inflammatory agent, piroxicam: modification of classical protocols. Toxicology. 1984;30(1):59-63.
You may like
Related articles And Qustion
Lastest Price from Piroxicam manufacturers

US $0.00-0.00/kg2025-05-29
- CAS:
- 36322-90-4
- Min. Order:
- 1kg
- Purity:
- 99% up by HPLC
- Supply Ability:
- 10tons

US $1.00/KG2025-04-21
- CAS:
- 36322-90-4
- Min. Order:
- 1KG
- Purity:
- 97%~103%,USP40
- Supply Ability:
- 500kg/month