Piroxicam: Pharmacological Properties, Therapeutic Efficacy and Synthesis
General Description
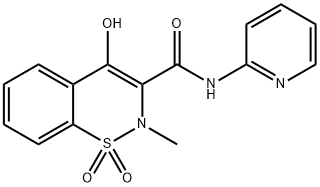
Piroxicam is an N-heterocyclic carboxamide of 1,2-benzothiazine-1,1-dioxide with analgesic and anti-inflammatory activity. It has an extended half-life of about 40 hours and is suitable for once daily administration. Published studies indicate that piroxicam 20mg daily is comparable with aspirin 3 to 6 g, indomethacin 75 to 150mg, phenylbutazone 400mg, naproxen 500mg, ibuprofen 1200 to 2400mg and diclofenac 75mg in rheumatoid arthritis . In osteoarthritis, piroxicam 20mg daily is comparable in efficacy with aspirin 2.6 to 3.9g, indomethacin 75mg, naproxen 500mg and fenbufen 600mg but is generally better tolerated than aspirin or indomethacin in patients with arthritic diseases. Piroxicam 20mg was at least as effective as indomethacin 75mg in a study in anyklosing spondylitis. As with other non-steroidal anti-inflammatory drugs gastrointestinal complaints are the most frequent ly reported side effects and their frequency and severity appears to be dose-related.1

Figure 1. Properties of Piroxicam Drug Substance
Pharmacology
Piroxicam is a new non-steroidal analgesic and anti-inflammatory agent unrelated chemically to other available drugs . In nonspecific animal models of inflammation its potency (weight. for-weight) is similar to that of indomethacin. As an analgesic piroxicam is more potent (weight-for-weight) than aspirin, fenoprofen , ibuprofen , naproxen or phenylbutazone in inhibiting phenylquinone-induced writhing . In preliminary studies, piroxicam 20mg daily caused less faecal blood loss than aspirin 3.8g daily in humans . Piroxicam, like many other non-steroidal anti-inflammatory drugs, inhibits the secondary phase of platelet aggregation induced by adenosine diphosphate and collagen-induced aggregation. In vitro and in vivo. piroxicam is an inhibitor of prostaglandin synthesis, being a selective reversible inhibitor of the cycle-oxygenase step of arachidonic acid metabolism.2-3
Pharmacokinetics
Piroxicam is readily absorbed from the oral or rectal routes and reaches steady-state after about 7 days. After repeated doses of 20mg daily (the usual therapeutic dose) for 2 weeks, mean plasma concentrations 48 hours after the last dose are still within the therapeutic range. Piroxicam penetrates into the synovial fluid of patients with rheumatoid arthritis and attains concentrations about 40%of those in plasma. On the basis of available data piroxicam appears to be extensively metabolised to inactive metabolites. The elimination half-life of piroxicam is extended due to a low clearance rate and has usually been calculated at around 38 hours in healthy subjects.4-5
Therapeutic Trials
In patients with rheumatoid arthritis piroxicam has been shown to be superior to placebo in practically all assessment criteria. Piroxicam 10mg twice daily was comparable with aspirin in maximum tolerated doses (2.65 to 6.3g daily) and 20mg once daily comparable with aloxiprin 4.8g daily. Studies comparing piroxicam with indomethacin, phenylbutazone and the phenylalkanoic or phenylacetic acid derivatives have generally indicated that piroxicam 20mg once daily or in divided doses, is comparable in efficacy with indomethacin 75 to 150mg, ibuprofen 1200mg daily, naproxen 500mg daily, diclofenac 75mg daily and phenylbutazone 400mg daily.
In patients with osteoarthritis, piroxicam 20mg daily appears to be at least as effective aspirin 2.6 to 3.9g daily, naproxen 500mg daily, or fenbufen 600mg at night and comparable with indomethacin 75mg daily in small numbers of patients. Long term studies in adequate numbers of patients demonstrated that the effectiveness of the drug is maintained without an increase in dosage and that at dosages of 20mg daily it is well tolerated by patients with osteoarthritis.
A comparative trial indicates that piroxicam 20mg is an alternative to indomethacin 75mg in ankylosing spondylitis. Open trials in acute gout have been promising, but further comparative trials are necessary to determine the role of piroxicam relative to that of established drugs.
In women with moderate to severe episiotomy pain, piroxicam 20 to 40mg was comparable with aspirin 648mg in providing pain relief. In acute musculoskeletal disorders doses of 40mg daily for the first 2 days, reducing to 20mg thereafter were comparable with phenylbutazone 600mg daily for 2 days and 300 to 400mg thereafter.6
Side Effects
The most frequently reported side effects have been gastrointestinal effects, but these have generally occurred less frequently with piroxicam than with therapeutically equivalent doses of aspirin , indomethacin or phenylbutazone . Peptic ulceration has occurred infrequently (I % overall incidence)at a dosage of 20mg daily, but has been 2 to 5 times more frequent at a dose of 40mg daily.7
Dosage and Administration
The initial adult dosage in rheumatoid arthritis, osteoarthritis and ankylosing spondylitis is 20mg daily. In most patients 20mg given as a single daily dose is adequate for long term maintenance, but a few may require 30mg daily. Higher dosages are associated with an increased risk of gastrointestinal side effects during extended periods of treatment. In acute gout an initial 40mg dose should be given followed by 40mg daily, as a single dose or in divided doses, for the next 4 to 6 days. A dose of 40mg daily is also used for the first 2 days of treatment of acute musculoskeletal disorders , reducing to 20mg daily for the remainder of the treatment period.6
Synthesis
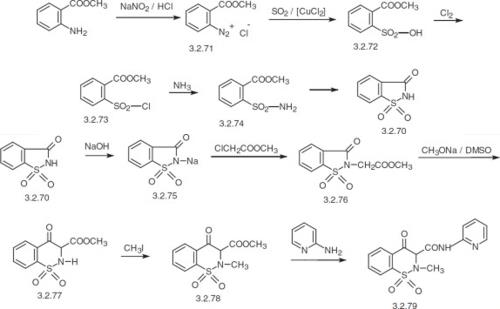
Figure 2. Preparation of Piroxicam
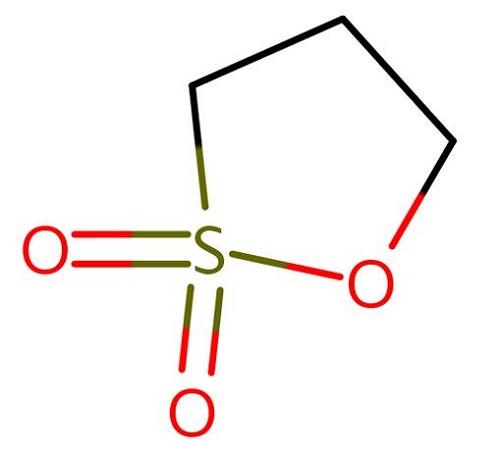
Piroxicam is synthesized from saccharin. Preparation of saccharin is from methyl ester o-aminobenzoic (anthranylic acid). This undergoes diazotization using nitrous acid, and the resulting diazonium salt is reacted with sulfur dioxide in the presence of copper dichloride, forming the methyl ester o-sulfobenzoic acid. Reaction of the resulting product with chlorine gives o-chlorosulfonylbenzoic acid methyl ester, which upon reaction with ammonium gives o-sulfonylamidobenzoic acid methyl ester. In the presence of hydrogen chloride, the resulting product undergoes cyclization into saccharin. The reaction of saccharin with sodium hydroxide results in substitution of the imide hydrogen atom of saccharin with sodium, giving a sodium salt. The resulting product is reacted with methyl chloroacetate, giving the saccharin-substituted acetic acid methyl ester. Upon reaction with sodium methoxide in dimethylsulfoxide, the product undergoes rearrangement into 1,1-dioxide 3-methoxycarbonyl-3,4-dihydro-2-H-1,2-benzothiazin-4-one. This product is methylated at the nitrogen atom using methyl iodide, giving. Finally, reaction of the resulting product with 2-aminopyridine gives piroxicam.8
References
1. Abe, T.: Piroxicam as a new adjunct for treatment for rheumatoid arthritis. American Journal of Medicine. 1981
2. Schiantarelli , P.; Acerbi, D. and Bovis, G.: Some pharmacokinetic properties and bioavailability by oral and rectal route of piroxicam in rodents and in man. Arzneimittel-Forschung 3I : 92-97 (1981)
3. Wiseman, EH. and Reinert, H.: Anti-inflammatory drugs and renal papillary necrosis. Agents and Actions 5: 322-325 (975).
4. Hobbs, D.C. and Twomey , T.M.: Piroxicam pharmacokinetics in man : Aspirin and antacid interaction studies. Journal of Clinical Pharmacology 19: 270-281 (979).
5. Mercier, M. and l..esne, M.: The pharmacokinetics of piroxicam and indomethacin; in Boyle (Ed.) Rheumatology in the Eighties; an Advance in Therapy - Piroxicam (Excerpta Medica, Princeton 1980).
6. Brogden, R. N., Heel, R. C., Speight, T. M., & Avery, G. S. (1984). Piroxicam: a reappraisal of its pharmacology and therapeutic efficacy. Drugs, 28, 292-323.
7. Pisko, E.J.; Rahman, M.A.; Turner, R.A. and Agudelo, CA.: Long term efficacy and safety of piroxicam in the treatment of rheumatoid arthritis. Current Therapeutic Research 27: 852-859 (1980).
8. Vardanyan, R., & Hruby, V. (2006). Synthesis of essential drugs. Elsevier.
Related articles And Qustion
See also
Lastest Price from Piroxicam manufacturers

US $0.00-0.00/kg2025-05-29
- CAS:
- 36322-90-4
- Min. Order:
- 1kg
- Purity:
- 99% up by HPLC
- Supply Ability:
- 10tons

US $1.00/KG2025-04-21
- CAS:
- 36322-90-4
- Min. Order:
- 1KG
- Purity:
- 97%~103%,USP40
- Supply Ability:
- 500kg/month