DM4:Activity and Preparation
General Description
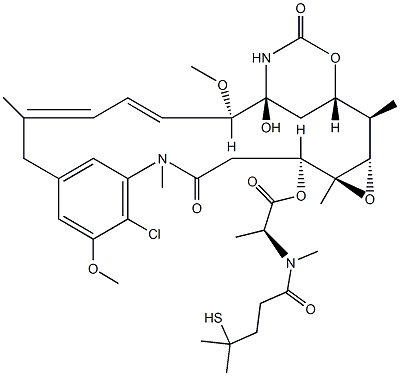
The DM4, as the cytotoxic agent refering to a substance that inhibits or prevents one or more cellular functions and/or causes cell death, which can be used in the preparation of antibody drug conjugate and it is an antitubulin agent. It can inhibit cell division.The DM4 is released within the cell and exerts its cytotoxic activity. DM4, a structural analogue of maytansine, is a new thiolcontaining and potent maytansinoid. It was synthesized in order to link maytansinoids to antibodies via disulfide bonds.[1-3]DM4 is a cytotoxic maytansinoid drug.[4]

Figure 1 DM4 particle
Activity
DM4, an antimitotic agent like other Vinca alkaloids, binds preferentially to α/β-tubulin heterodimers and inhibits tubulin polymerization and microtubule assembly, increasing microtubule destabilization, which leads to a potent suppression of microtubule dynamics and results in mitotic block and subsequent apoptotic cell death.[1]Maytansinoid DM4 is a thiol-containing maytansine derivative with highly potent cytotoxicity. Maytansinoid DM4 can be used as a cytotoxic moiety of ADC.[6-7]
Preparation
Maytansine is a natural benzoansamacrolide product isolated from the bark of the African shrub Maytenus ovatus. Maytansine binds to the same site on tubulin as the vinca alkaloids, with similar in vitro inhibition constants, but is a more-potent cytotoxin.[6]Isobutylene sulfide is reacted with the anion of acetonitrile to give the mercapto compound 6. Hydrolysis of 6 with base provided 4-mercapto-4-methylpentanoic acid. Conversion of 7 into disulfide 8 is achieved by reaction with methyl methanethiolsulfonate (MeSSO2Me). Conversion of 8 into the N-hydroxysuccinimide ester followed by reaction with N-methyl-L-alanine provided the carboxylic acid 10, which was purified by column chromatography over silica gel. Reaction of N-methyI-N-(4-metliyl-4-inethyldithio-l-oxopentyl)-L-aIanine with maytansinol in the presence of N,N’-dicyclohexylcarbodiimide (DCC) and zinc chloride gave a mixture of the N-acyl-N-methyl-L-alanyl maytansinoid L-DM4SMe, (4e) and the N-acyl-iV-methyl-D-alanyl maytansinoid D-DM4SMe. The mixture of diastereomers was separated by HPLC1 using a cyano-bonded column. The desired L-amino acid-containing isomer 4e was collected and reduced with dithiothreitol to give the thiol-containing L-aminoacyl maytansinoid DM4, which was again purified by HPLC, using a cyano-bonded column.The specific steps are as follows:
Step one:The preparation for 4-Mercapto-4-methylpentanoic acid (7): A 500 mL flask was equipped with a stir bar and a 150 mL addition funnel. The system was placed under an argon atmosphere. 150 mL of anhydrous tetrahydrofurane (THBF) and 75 mL of 2.5 M n-BuLi in hexanes (18.7 mmol) were added via a canula and the solution was cooled in a -78℃. dry ice/acetone bath. Acetonitrile (7.3 g, 9.4 mL, 18 mmol) was added drop-wise via a syringe over approximately 5 min. The reaction was stirred for 30 min, while white lithiumacetonitrile precipitate was formed. Isobutylene sulfide (15 g, 17 mmol) was dissolved in 100 m L of anhydrous THF and added drop wise over approximately 30 min via the addition funnel. The cooling bath was removed and the reaction was allowed to stir for 3 hours. The flask was cooled in an ice/water bath as 38 mL of 0.5 M HCl was added drop-wise. The THF layer was retained and the aqueous layer was washed twice with 75 mL of ethyl acetate. The THF and ethyl acetate layers were combined, dried over approximately 20 g of anhydrous sodium sulfate and transferred to a 250 mL flask. Solvent was removed by rotary evaporation under vacuum to give crude 6. Ethanol (30 mL) and a stir bar were added. The contents were stirred as a solution of 8.0 g NaOH in 30 mL deionized water was slowly added. The flask was equipped with a reflux condenser and placed under an argon atmosphere. The reaction was refluxed overnight then cooled to room temperature. Deionized water (60 mL) was added and the mixture was extracted twice with 25 mL portions of a 2:1 mixture of ethyl acetate and hexane. The aqueous layer was acidified to pH 2 with concentrated HCl then extracted three times with 75 mL portions of ethyl acetate. The organic layers were dried over anhydrous NajSO4 and solvent was removed by rotary evaporation under vacuum to give 10 g of product 7 (39% yield). Material was used without further purification.
Step two:4-Methyl-4-(methyldithio)pentanoic acid (8): A solution of mercaptopentanoic acid 7 (6.0 mL, 40 mmol) was dissolved in 50 mL of deionized water in a 250 mL flask. The solution was magnetically stirred as sodium carbonate (6.4 g, 60 mmol) was added to the acid at a rate that would not cause excessive frothing. The flask was equipped with a 100 mL addition funnel, which was charged with a solution of methyl methanethiolsulfonate (7.5 g, 60 mmol) dissolved in 30 mL of glass-distilled 100% ethanol. The flask was cooled in an ice/water bath and the system was maintained under an argon atmosphere. The methyl methanethiolsulfonate solution was added drop-wise to the flask as rapidly as possible but without causing excessive frothing. The cooling bath was removed and the reaction mixture was allowed to stir for an additional 3 hours. Solvent was removed by rotary evaporation under vacuum, until approximately 20 mL remained. After which 10 mL of saturated sodium bicarbonate and 30 mL of deionized water were added. The mixture was washed three times with 25 mL portions of ethyl acetate in a separatory funnel. The aqueous layer was adjusted to approximately pH 2 with 5 M HCl and was extracted twice with 120 mL portions of ethyl acetate. The organic layers were combined and washed with 20 mL of a solution composed of saturated NaCl and IM HCl at a ratio of 4:1. The organic layer was then dried over 14 g of anhydrous sodium sulfate and solvent was removed by rotary evaporation under vacuum to give 5.4 g of product 8 (70% yield). The material can be taken to the next step without further purification.
Step three: N-Hydroxysuccinimidyl 4-methyl-4-(methyldithio)pentanoate (9): Methyldithiopentanoic acid 8 (3.0 g, 15 mmol) was dissolved in 20 mL of methylene chloride and stirred magnetically as N-hydroxysuccinimide (2.65 g, 23 mmol) was added followed by l-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride (EDC, 4.4 g, 23 mmol). The mixture was stirred under an argon atmosphere for 2 hours. The reaction mixture was poured into a 125 mL separatory funnel, 40 mL of ethyl acetate was added and the solution was washed twice with 20 mL portions of 50 mM potassium phosphate buffer, pH 6.0, and once with 12 mL of saturated sodium chloride. The organic layer was dried over 14 g of anhydrous Na2SO4 and solvent was removed by rotary evaporation under vacuum to give 4.0 g of product 9 (90% yield), which was used without further purification.
Step four:N-methyl-N-(4-methyl-4-methyldithio-l-oxopentyl)-L-alanine (10): N-Methyl-L-alanine (2.85 g, 18.0 mmol) was dissolved in 50 mL of a 1:1 solution of dimethoxyethane and deionized water in a 125 mL flask equipped with a magnetic stir bar. Triethylamine (6.9 g, 36 mmol) was added and the solution was vigorously stirred as 9 (5.44 g, 18 mmol) dissolved in 40 mL of the same solvent mixture was added drop-wise over approximately 5 min. After 2 hours the reaction mixture was concentrated to approximately 40 mL by rotary evaporation under vacuum, then 10 mL of deionized water and I M HCl were added to give a pH of approximately 2. The mixture was poured into a separatory funnel and extracted twice with 50 mL portions of ethyl acetate. The organic layers were combined and then washed with 7 mL of saturated sodium chloride solution. The organic layer was dried over 8.0 g of anhydrous Na2SO4 and the solvent was removed by rotary evaporation under vacuum. The residue was taken up in a minimum volume of ethyl acetate and purified by chromatography on silica (silica: 40 micron flash grade, silica bed: 24x3.0 cm, mobile phase: hexanes:ethyl acetate:acetic acid 50:48:2). Fractions containing desired product were combined and solvent was removed under vacuum. Residual acetic acid was removed by dissolving the residue in a minimum volume of ethyl acetate and precipitating product by the rapid but drop-wise addition of hexane with stirring. Hexane was added until product was no longer detected in the supernatant by TLC analysis. The precipitate was vacuum dried for 4 hours to give 2.2 g of product 10 (51% yield).
Step five:N2’-deacetyl-N2’-(4-methyl-4-methyldithio-l-oxopentyl)maytansine (L-DM4-SMe, 4e). A solution of maytansinol (11,25 mg, 0.44 mmol) and N-methyl-N-(4-methyl4-methyldithio-l-oxopentyl)-L-alanine (10, 42.0 mg, 0.177 mmol) in 3 mL dichloromethane was magnetically stirred under an argon atmosphere as a solution of dicyclohexylcarbodiimide (DCC, 57.1 mg, 0.277 mmol) in 0.67 mL dichloromethane was added. After I min a solution of I M ZnCl2 in diethyl ether (0.03 mL, 0.03 mmol) was added. The mixture was stirred at room temperature for 2 hours then 5 mL of ethyl acetate was added and the mixture was vacuum filtered through course filter paper. The filtrate was washed with 2 mL of saturated sodium bicarbonate solution followed by I mL of saturated sodium chloride solution. The organic layer was dried over 2 g of anhydrous sodium sulfate. And solvent was removed under vacuum and the residue was purified by silica chromatography using a mixture of dichloromethane and methanol to remove unreacted maytansinol. Fractions containing desired product were combined and solvent was removed under vacuum to give a mixture of diastereomers 4e and 4f. The residue was taken up in a minimum volume of ethyl acetate and purified on a 50 cm by 250 cm, 10 micron Diazem™ CN column using as mobile phase a mixture of hexane, 2-propanol and ethyl acetate at a ratio of 68:8:24. The flow rate was 118 mL/min. Under these conditions the desired product 4e eluted with a retention time of 11 min and the undesired diastereomer 4f had a retention time of 19 min. Fractions containing desired product were combined and solvent was removed under vacuum to give 12.0 mg of product 4e (36% yield).
Step six:N2 -deacetyl-N2 -(4-mercapto-4-methyl-l-oxopentyl)maytansine (L-DM4, 4b). Uie disulfide 4e from above (12 mg, 0.015 mmol) was dissolved in 1.0 mL of 1:1 ethyl acetate:methanol. A solution of dithiothreitol (18 mg, 0.117 mmol) in 0.50 mL of 50 mM phosphate buffer, pH 7.5, was then added. The solution was magnetically stirred under an argon atmosphere for 3 hours, then I mL of 200 mM phosphate buffer, pH 6.0, was added and the mixture was extracted three times with 2 mL portions of ethyl acetate. The organic layers were combined and washed with I mL of saturated sodium chloride solution, then dried over I g of anhydrous sodium sulfate. The solvent was removed under vacuum and the residue was taken up in a minimum of ethyl acetate and purified on a 50 cmx250 cm, 10 micron Diazem™ CN column using as mobile phase a mixture of hexane, 2-propanol and ethyl acetate at a ratio of 70:8:22. The flow rate was 22 mL/min. Hie desired product 4b eluted with a retention time of 10 min. Fractions containing pure 4b were combined and the solvent was removed under vacuum to give 11 mg of DM4(4b) (97% yield).[5]
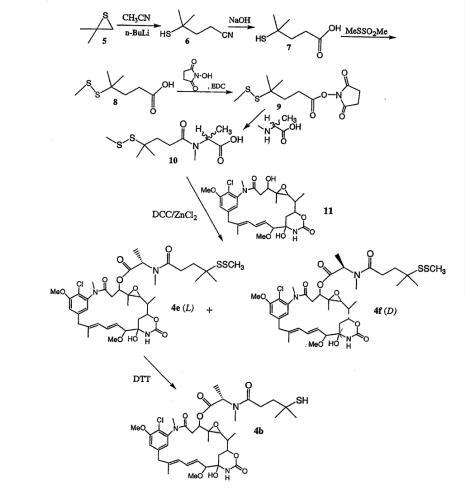
Figure2 DM4 synthesis
Reference
[1]Remillard S, Rebhun LI, Howie GA, Kupchan SM: Antimitotic activity of the potent tumor inhibitor maytansine[J]. Science 1975, 189(4207):1002-5.
[2]Chari R, Zhang W, Meshulam D, et al. Process for preparing stable drug conjugates: U.S. Patent Application 11/352,121[P]. 2006-8-17.
[3]Westin, Eric, Wang, Jiuzhou, et al. A biparatopic antibody drug conjugate targeting alpha folate receptors testing safety and efficacy for solid tumor immunotherapy in women.[P]WO 2022/256507.
[4]Tang R, et al. P-gp activity is a critical resistance factor against AVE9633 and DM4 cytotoxicity in leukaemia cell lines, but not a major mechanism of chemoresistance in cells from acute myeloid leukaemia patients[J]. BMC Cancer. 2009,23,9:199.
[5]Chari RVJ, Widdison, WC. Preparation of cytotoxic agents comprising new maytansinoids[P].U.S. Pat. Appl. Publ.2004, US20040235840.
[6]Beck A, et al. Strategies and challenges for the next generation of antibody-drug conjugates[J]. Nat Rev Drug Discov. 2017,16(5):315-337.
[7]Zhao RY, et al. Synthesis and evaluation of hydrophilic linkers for antibody-maytansinoid conjugates[J]. J Med Chem. 2011,26;54(10):3606-23.