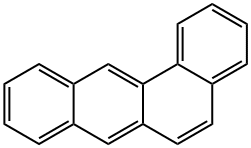
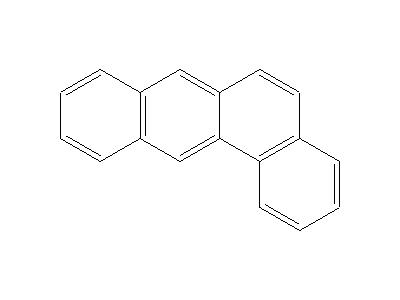
What is 1,2-benzanthracene?
Fig 1. Chemical structure formula of 1,2-benzanthracene
1,2-Benzanthracene (C18H12, CAS registry No. 56-55-3) is colorless leaflets or plates or coarse gold powder with a greenish-yellow fluorescence. Its melting point is 159.8 oC and flash point is 209.1 oC. It is nearly insoluble in water and the solubility is only 0.0000014g in 100 mL water. It is stable under normal temperatures and pressures. It is found in oils, waxes, smoke, food, drugs. However, it is combustible and incompatible with strong oxidizing agents. It may react vigorously with strong oxidizing agents. It can react exothermically with bases and with diazo compounds. Substitution at the benzene nucleus occurs by halogenation (acid catalyst), nitration, sulfonation, and the Friedel-Crafts reaction. When heated to decomposition, it emits acrid smoke and irritating fumes. Under fire conditions, hazardous decomposition products carbon oxides are formed.
It may reasonably be expected that 1,2-benzanthracene is a carcinogen[1] with experimental carcinogenic, neoplastigenic, and tumorigenic data by skin contact and other routes. It is very toxic to aquatic life with long lasting effects. The effect of different concentrations of 1,2-benzanthracene on ultrastructural organization of plant cell has been studied[2]. It has been shown that low concentrations of 1,2-benzanthracene (20-100 ~μg/mL) does not cause noticeable changes on ultrastructural level due to the cell’s ability to assimilate and metabolize them. Higher concentrations of 1,2-benzanthracene (200 ~μg/mL) leads to significant ultrastructural changes up to the complete destruction of the cell.
1,2-Benzanthracene is a polycyclic aromatic hydrocarbons (PAH). Many methods have been developed for the synthesis of 1,2-benzanthracene and 1,2-benzanthracene derivatives. A facile and efficient protocol for the synthesis of 1,2-benzanthracene derivatives was the manganese (III) acetate-mediated free radical cyclization of diarylmethylenecyclopropa[b]naphthalenes with nucleophiles such as carboxylic acid and sulfonic acid[3]. This efficient method can obtain 1,2-benzanthracenes in moderate to good yields under mild conditions. In addition, after several steps of simple and routine operations, the obtained 1,2-benzanthracenes bearing an acetoxy group could be easily converted to structurally more sophisticated 1,2-benzanthracene derivatives, which are not easily accessible yet potentially useful candidates for materials science.
References
[1]Steiner, P. E.; Edgcomb, J. H., Carcinogenicity of 1,2-Benzanthracene. Cancer Research 1952, 12 (9), 657-659.
[2]Buadze, O.; Sadunishvili, T.; Kvesitadze, G., The effect of 1,2-benzanthracene and 3, 4-benzpyrene on the ultrastructure of maize cells. International Biodeterioration & Biodegradation 1998, 41 (2), 119-125.
[3]Cao, J.; Miao, M.; Chen, W.; Wu, L.; Huang, X., Manganese(III) Acetate-Mediated Cyclization of Diarylmethylenecyclopropa[b]naphthalenes: A Method for the Synthesis of 1,2-Benzanthracene Derivatives. The Journal of Organic Chemistry 2011, 76 (22), 9329-9337.
[4] http://www.chemspider.com/Chemical-Structure.5739.html
You may like
Related articles And Qustion
See also
Lastest Price from Benz[a]anthracene manufacturers
![56-55-3 Benz[a]anthracene](/ProductImageEN/2024-07/Small/e2da73fc-2e4a-47f7-9e86-f3956c4b7d99.gif)
US $0.00-0.00/Kg2025-04-12
- CAS:
- 56-55-3
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 100kg