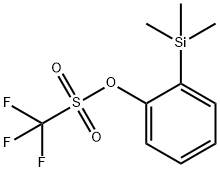
How to prepare 2-(trimethylsilyl)phenyl trifluoromethanesulfonate?
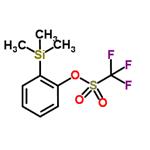
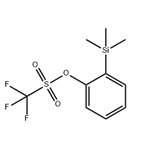
2-(Trimethylsilyl) phenyl trifluoromethanesulfonate (C10H13F3O3SSi, CAS: 88284-48-4) [1] is an important ortho-benzyne precursor in aryne chemistry. 2-(Trimethylsilyl) phenyl trifluoromethanesulfonate may be used to generate benzyne under mild conditions of simple fluoride treatment at room temperature [2]. But did you hear about how 2-(trimethylsilyl)phenyl trifluoromethanesulfonate itself come into being? Here, we present a method for the efficient synthesis of this important ortho-benzyne precursor?
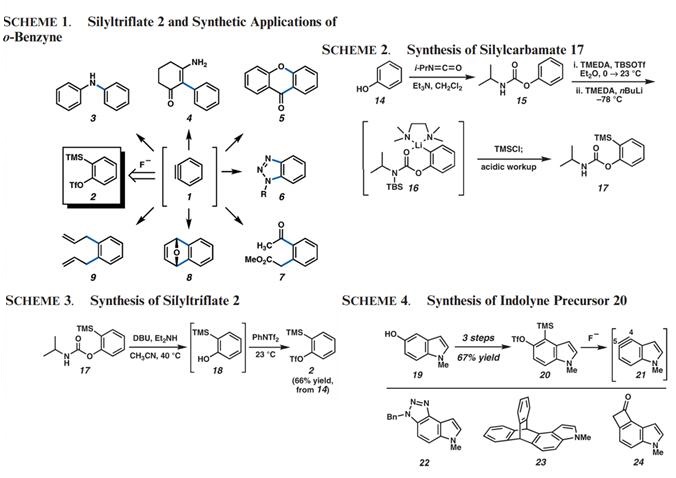
o-Benzyne (1) has garnered much attention as a valuable synthetic building block (Scheme 1). Although a number of methods for benzyne generation have been discovered, Kobayashi’s mild fluoride-induced formation of benzyne (1) from 2-(trimethylsilyl) phenyl trifluoromethanesulfonate (2) has proven most versatile. The use of 2 as a precursor to benzyne has enabled the synthesis of substituted aromatic compounds and has spawned the discovery of many new chemical reactions (e.g., 1 f 3-9).
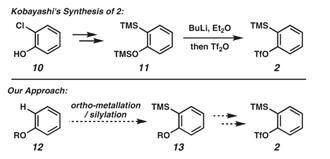
Despite the importance of 2-(trimethylsilyl) phenyl trifluoromethanesulfonate (2) in modern organic synthesis, literature procedures for its preparation are sparse. In Kobayashi’s original report, the benzyne precursor was synthesized from o-chlorophenol (10) by a sequence involving conversion to bis(silylated) intermediate 11, followed by lithiation/triflation (Figure 1). A recent paper describes a synthesis of 2 in three steps from 2-bromophenol, using an analogous strategy. We envisioned that an alternative synthesis of silyltriflate 2 could be implemented by using phenol as the starting material. In this approach, ortho-metalation of a suitably protected phenol derivative would provide a means to introduce the necessary trimethylsilyl substituent (12 f 13) en route to the coveted benzyne precursor 2.
The pioneering studies by Snieckus in the area of directed metalation chemistry led us to examine the use of arylcarbamates as intermediates in our planned synthesis of 2. Although both N,N-dialkylcarbamate and N-monoalkyl carbamate derivatives of phenol could easily be prepared and utilized for the desired metalation/silylation, we elected to focus on monoalkyl derivatives which would be more readily cleaved at a later stage. Thus, phenol (14) was allowed to react with isopropyl isocyanate in the presence of cat. Et3N to provide carbamate 15 in quantitative yield (Scheme 2). Using the one-pot procedure developed by Snieckus and Hoppe, crude 15 underwent N-silylation, followed by ortho-lithiation to provide intermediate 16, which in turn was quenched with TMSCl to install the necessary trimethylsilyl substituent. After workup, the desired silylcarbamate 17 was obtained without the need for chromatographic purification. Notably, this robust protocol could be carried out reliably on multigram scale.
To access the desired silyltriflate, a number of methods for carbamate cleavage/triflation were explored. Although stepwise routes provided initial success, we ultimately uncovered a one-pot procedure that allowed for the conversion of carbamate 17 to silyltriflate 2 (Scheme 3). Exposure of carbamate 17 to DBU and Et2NH in CH3CN at 40 ℃ furnished intermediate o-silylphenol 18. After the reaction mixture was cooled to room temperature, a solution of PhNTf2 in CH3CN was introduced to facilitate triflation. Following purification by flash chromatography, silyltriflate 2 was obtained as a colorless oil in 66% yield, over the three steps. With use of this sequence, 5 g of phenol can be smoothly converted to>10 g of 2. It is expected that our method for the conversion of phenol to silyltriflate 2 will be amenable to the synthesis of other aryne precursors. For instance, our laboratory has utilized the methodology to elaborate hydroxyindole 19 to indolylsilyltriflate 20 (Scheme 4) in 67% yield over three steps. Silyltriflate 20 provides a means to generate indolyne 21, which in turn serves as a valuable precursor to a variety of novel indole derivatives (e.g., 22-24).
In summary, we have developed an efficient procedure for the gram-scale preparation of 2-(trimethylsilyl)phenyl trifluoromethanesulfonate, a versatile precursor to o-benzyne. The three-step sequence utilizes phenol as the starting material, requires only one chromatographic purification, and ultimately delivers silyltriflate 2 in 66% overall yield. We expect the method will also prove amenable to the synthesis of other aryne precursors.
References
[1] https://pubchem.ncbi.nlm.nih.gov/compound/3384007
[2] https://www.sigmaaldrich.com/catalog/product/aldrich/470430?lang=en®ion=GB
[3] Bronner, S. M. , and N. K. Garg . "Efficient synthesis of 2-(trimethylsilyl)phenyl trifluoromethanesulfonate: a versatile precursor to o-benzyne." Journal of Organic Chemistry74.22(2009):8842-3.
[4] http://www.chemspider.com/Chemical-Structure.2629091.html?rid=a2cb18ab-0b30-4bbc-a67e-717eca5b7ef7
You may like
Lastest Price from 2-(TRIMETHYLSILYL)PHENYL TRIFLUOROMETHANESULFONATE manufacturers
US $2.20-8.80/kg2025-09-08
- CAS:
- 88284-48-4
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 100kg
US $0.00-0.00/kg2025-04-04
- CAS:
- 88284-48-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton