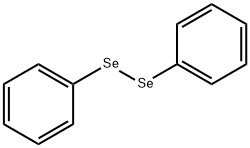
What happens when Diphenyl diselenide encounters Nucleophilic Phenylseleno Reagents or Electrophilic Phenylseleno Reagen
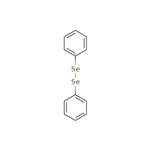
Diphenyl diselenide(CAS: 1666-13-3) is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2 [1]. This orange-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis [2].
Incorporation of Nucleophilic Phenylseleno Reagents.
Diphenyl diselenide and Benzeneselenol are the most popular reagents for the generation of nucleophilic phenyl selenide. Diphenyl diselenide is usually the preferred precursor due to its greater ease in handling. Selenolate anions are potent, soft nucleophiles.
There are many popular and generally applicable procedures for the generation of benzeneselenolate anions from PhSeSePh. Treatment of PhSeSePh with Sodium Borohydride provides a very convenient method for the generation of the benzeneselenolate anion. The nucleophilicity of the anion thus formed is apparently decreased due to complexation with borane. Uncomplexed sodium benzeneselenolate can be generated upon reduction of PhSeSePh with Sodium Hydride5 or Sodium metal. Generation of sodium benzeneselenolate upon treatment of PhSeSePh with Rongalite (Sodium Hydroxymethanesulfinate) has been mentioned as being particularly amenable to large scale preparations, due to the absence of H2 evolution.
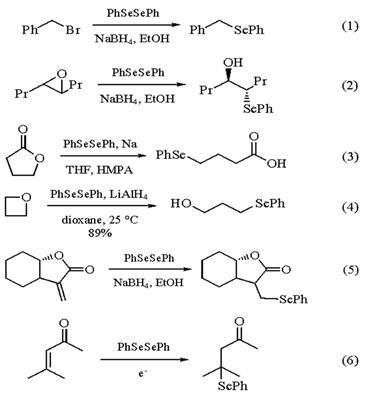
The benzeneselenolate anion is reactive with a variety of carbon electrophiles. It is reactive with benzyl (eq 1) and alkyl halides, leading to the formation of simple alkyl phenyl selenides. The benzeneselenolate anion is also useful in the nucleophilic ring opening of oxiranes (eq 2). This method can be of limited value with unsymmetrical oxiranes, as regioselectivity is often poor. While the borane-complexed benzeneselenolate anion generated from treatment of PhSeSePh with NaBH4 is not a reactive enough nucleophile to cleave esters, a variety of more reactive benzeneselenolate anions are quite effective in this regard (eq 3). A particularly reactive form can be formed by reaction of PhSeSePh with Lithium Aluminum Hydride. This species is capable of reaction with oxetanes and oxolanes, cleaving the ethereal carbon-oxygen bond (eq 4).
The benzeneselenolate anion also adds in a conjugate fashion to unsaturated carbonyl compounds. Addition to a-methylene lactones proceeds quite readily (eq 5). Given the facility with which subsequent oxidative elimination of the phenylseleno group can be carried out, thus regenerating unsaturation, an effective means of protecting a labile a-methylene lactone is available. The benzeneselenolate anion can also be generated electrochemically and added to unsaturated ketones and esters in a conjugate fashion (eq 6).
Incorporation of Electrophilic Phenylseleno Reagents.
A variety of carbanion nucleophiles react with PhSeSePh to generate the corresponding phenylseleno-substituted product. While PhSeSePh is not as electrophilic as other organoselenium reagents, such as Benzeneselenenyl Chloride, Benzeneselenenyl Bromide, or N-Phenylselenophthalimide, there are numerous situations where it can be used to supply electrophilic phenyl selenide.
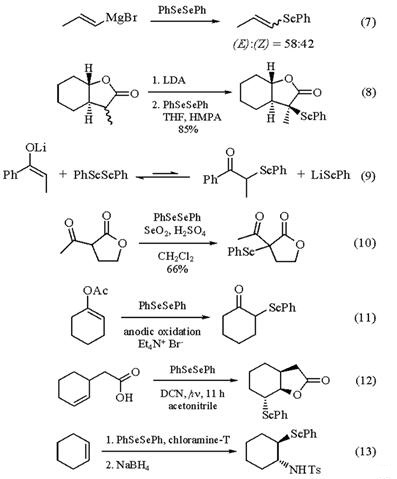
Diphenyl diselenide reacts readily with many organometallic species (eq 7).Similar reactivity has also been observed with ester and lactone enolates (eq 8) and nitrile-stabilized carbanions.The reaction of ketone enolates with PhSeSePh usually fails due to an unfavorable equilibrium constant (eq 9). However, this unfavorable equilibrium can be driven to completion upon addition of O2, which converts the PhSe- formed in the reaction to PhSeSePh.
Methods involving the reaction of less reactive nucleophiles with PhSeSePh usually involve in situ generation of the phenylselenium cation, or some other highly reactive phenyl selenide species. A variety of dialkyl ketones, aliphatic aldehydes, and even b-keto esters or b-diketones react with PhSeSePh in the presence of Selenium(IV) Oxide and a trace of H2SO4 (eq 10). Enol acetates react with PhSeSePh upon electrochemical anodic oxidation in the presence of Et4N+Br- (eq 11). It has been proposed that this method involves the in situ generation of PhSeBr. Phenyl selenoacetate is probably generated in situ upon reaction of PhSeSePh with Copper(II) Acetate or Lead(IV) Acetate. This reagent effects the trans-acetoxyselenation of alkenes. The phenylselenium cation has been generated from PhSeSePh upon photolysis in the presence of DCN, proceeding through a mechanism involving photoinduced electron transfer (eq 12), or upon treatment of PhSeSePh with (NH4)2S2O8. The phenylselenium cation thus generated induces selenolactonization and selenoetherification. Stereospecific selenoamination, proceeding through the possible intermediacy of a selenonium ion, has been reported upon reaction of cyclohexene with PhSeSePh in the presence of Chloramine-T (eq 13) [3].
References
[1] Wikipedia: diphenyl diselenide
[2] https://pubchem.ncbi.nlm.nih.gov/compound/Diphenyl-diselenide
[3] http://reag.paperplane.io/00001231.htm
[4] http://www.chemspider.com/Chemical-Structure.14710.html?rid=139cd3ee-810e-4d16-a65d-d23253232007
You may like
Lastest Price from Diphenyl diselenide manufacturers
US $0.00/kg2025-04-15
- CAS:
- 1666-13-3
- Min. Order:
- 200kg
- Purity:
- 94%
- Supply Ability:
- 20 tons
US $780.00/Kg2024-02-25
- CAS:
- 1666-13-3
- Min. Order:
- 1Kg
- Purity:
- 99 %
- Supply Ability:
- 500 Kg