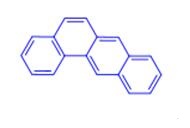
Benz[a]anthracene:Mechanism of Toxicity
A procarcinogen, benz[a]anthracene is metabolized to its
carcinogenic form by phase 1 and phase 2 metabolism. As
with other polycyclic aromatic hydrocarbons (PAHs), the
presence of the ‘bay’ region contributes greatly to the carcinogenicity
of benz[a]anthracene metabolites. This region is
sterically constrained, allowing the formation of diol epoxides,
which subsequently react with intracellular molecules
such as DNA.
Human exposure to benz[a]anthracene and other PAHs
occurs primarily from smoking or second-hand smoke, air
polluted with combustion products, or food and water
polluted with combustion products.
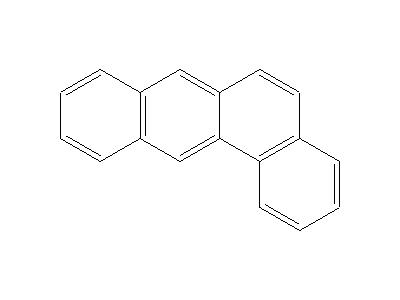
Uses
Benz[a]anthracene is primarily used in research.
Environmental Fate
Benz[a]anthracene is not synthesized commercially. The primary source of many PAHs in air is the combustion of wood and other fuels. PAHs released into the atmospheremay deposit onto soil or water. In surface water, PAHs can volatilize, bind to suspended particles, or accumulate in aquatic organisms. Adsorption to solid particles in the soil extended their half-life, benz[a]anthracene’s half-life in Kidman sandy loam is 261 days. The vapor pressure of benz[a]anthracene is 1.9×106mmHg at 25°C, and it has an atmospheric half-life of about 7.7 h due primarily to photochemical degradation.
Mechanism of Toxicity
While not toxic itself, benz[a]anthracene and other PAHs containing bay regions are carcinogenic. These compounds are metabolized by cytochrome P450 enzymes in collaboration with epoxide hydrolase to highly reactive diol epoxides. Thesemetabolites in turn covalently bind to nucleophilic sites in DNA and other biological molecules. Benz[a]anthracene may also be metabolized to radical cations that form depurinated DNA adducts.
You may like
Related articles And Qustion
See also
Lastest Price from Benz[a]anthracene manufacturers
![56-55-3 Benz[a]anthracene](/ProductImageEN/2024-07/Small/e2da73fc-2e4a-47f7-9e86-f3956c4b7d99.gif)
US $0.00-0.00/Kg2025-04-12
- CAS:
- 56-55-3
- Min. Order:
- 1Kg
- Purity:
- 98%
- Supply Ability:
- 100kg
![56-55-3 benz[a]anthraceneUsesEnvironmental FateMechanism of Toxicity](https://www.chemicalbook.com/CAS/GIF/56-55-3.gif)