Uses of Lithium bromide
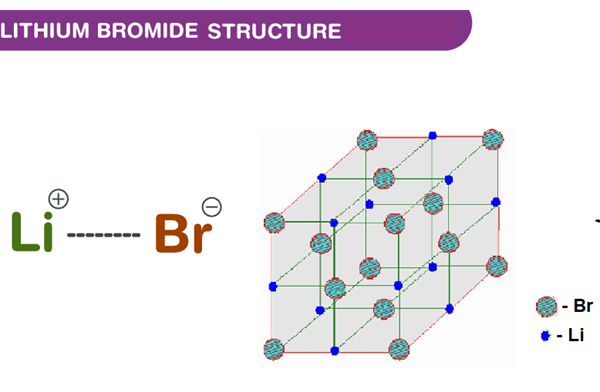
Lithium bromide is a lithium salt in which the counterion is bromide. The anhydrous salt forms cubic crystals similar to common salt. It is a bromide salt and a lithium salt.Lithium bromide is a chemical compound of lithium and bromine. Its extreme hygroscopic character makes LiBr useful as a desiccant in certain air conditioning systems. Lithium bromide (LiBr), also known as lithium monobromide, is a compound of the elements lithium and bromine, having one atom of lithium bonded to one atom of bromine. It is an ionic compound, meaning that the lithium atom “gives” one electron to the bromine atom, with the result that the lithium atom becomes positively charged and the bromine atom negatively charged; the atoms are then bonded together by electrostatic attraction. Ionic bonding is a common feature of simple metal/non-metal compounds.
Lithium is a metal belonging to a group of elements known as the alkali metals because they react with water to produce strong alkalis. Bromine belongs to a group of reactive non-metallic elements known as the halogens, which also include fluorine, chlorine and iodine. Lithium Bromide is a moderately water soluble crystalline Lithium source that decomposes to Lithium oxide on heating. Most metal bromide compounds are water soluble for uses in water treatment, chemical analysis and in ultra high purity for certain crystal growth applications. The bromide ion in an aqueous solution can be detected by adding carbon disulfide (CS2) and chlorine. Lithium Bromide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.
Fig 1. Chemical structure formula and three-dimensional structure of Lithium bromide
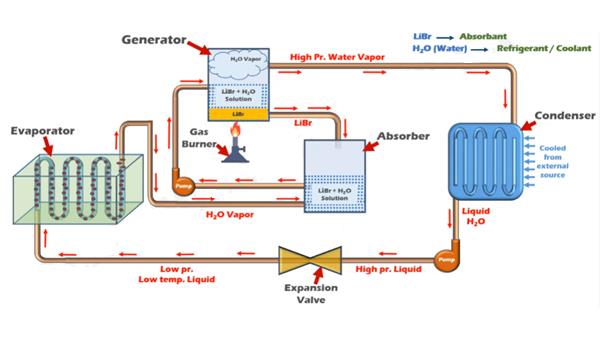
In a water-lithium bromide vapor absorption refrigeration system, water is used as the refrigerant while lithium bromide (Li Br) is used as the absorbent. In the absorber, the lithium bromide absorbs the water refrigerant, creating a solution of water and lithium bromide. This solution is pumped by the pump to the generator where the solution is heated. The water refrigerant gets vaporized and moves to the condenser where it is cooled while the lithium bromide flows back to the absorber where it further absorbs water coming from the evaporator. The water-lithium bromide vapor absorption system is used in a number of air conditioning applications,which is useful for applications where the temperature required is more than 32 degree F[1].
Lithium compounds affect central nervous system (CNS) functions and have been used to treat certain mental conditions, especially bipolar disorder. In modern times, lithium carbonate is normally prescribed, but lithium bromide has been used in the past for the treatment of epilepsy. Ingestion of lithium compounds can produce serious toxic effects because of their action on the CNS.
Lithium bromide (LiBr) can be used as a catalyst for the following studies[2-5]:
• Conversion of (aromatic- and α,β-unsaturated) aldehydes to dithioacetals by a solvent-free dithioacetal reaction.
• Synthesis of olefins by condensation of a carbonyl compound with an active methylene compound.
• Green synthesis of β-amino alcohol.
• Chemical and regioselective bromination of aromatics by using a LiBr/ammonium ammonium nitrate (CAN) reagent system (as a source of Br+).
References
[1] Principles of Refrigeration by Roy J. Dossat, 4th edition, Prentice Hall.
[2] Lithium bromide-catalyzed highly chemoselective and efficient dithioacetalization of α,β-unsaturated and aromatic aldehydes under solvent-free conditions. Firouzabadi H, et al. Synthesis , 58-60, (1999).
[3] An efficient chemo and regioselective oxidative nuclear bromination of activated aromatic compounds using lithium bromide and ceric ammonium nitrate. Roy SC, et al. Tetrahedron Letters 42(39), 6941-6942, (2001).
[4] Lithium bromide as a new catalyst for carbon-carbon bond formation in the solid state. Prajapati D, et al. Journal of the Chemical Society. Perkin Transactions 1 9, 959-960, (1996).
You may like
Related articles And Qustion
See also
Lastest Price from Lithium bromide manufacturers

US $0.10/KG2025-09-14
- CAS:
- 7550-35-8
- Min. Order:
- 1KG
- Purity:
- 99.9%+
- Supply Ability:
- 2000 kgs

US $10.00/KG2025-04-21
- CAS:
- 7550-35-8
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt