Lithium bromide: properties and safety
General Description
Lithium bromide is a compound composed of lithium and bromine. It has several distinctive properties that make it useful in various applications. Lithium bromide exhibits hygroscopic properties, making it effective as a liquid desiccant. It rapidly absorbs water vapor in the air, aiding dehumidification and refrigeration processes. Despite its usefulness, lithium bromide is corrosive and can damage materials, especially when exposed to moisture. Precautions must be taken when handling it, using corrosion-resistant materials in systems and following safety guidelines. Additionally, lithium bromide poses health risks if mishandled, with potential effects on the central nervous system and respiratory organs.

Figure 1. Lithium bromide
Properties
Hygroscopic nature
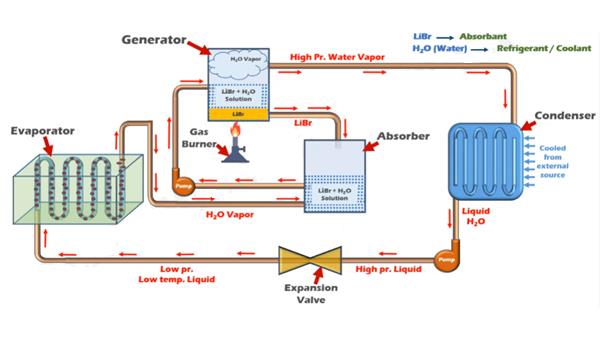
Lithium bromide exhibits hygroscopic properties as a liquid desiccant. A systematic experimental study was conducted on the vapor absorption of water into lithium bromide droplets at the microscale. The behavior of droplets on hydrophobic PTFE and hydrophilic glass substrates was investigated under controlled ambient conditions. The results showed that droplets on hydrophilic substrates demonstrated higher absorption kinetics and reached equilibrium with the ambient air faster than those on hydrophobic substrates. This was attributed to solute diffusion characteristics and the spreading process on different substrates. Overall, these findings provide insights into the hygroscopic nature of lithium bromide and its potential applications in dehumidification and refrigeration processes. 1
High solubility
Lithium bromide is known for its high solubility in water. It has one of the highest solubilities among alkali metal halides. At room temperature, approximately 1430 grams of lithium bromide can dissolve in one liter of water. This high solubility is due to the strong interaction between lithium cations (Li+) and bromide anions (Br-) with water molecules through ion-dipole interactions. These interactions lead to the dissociation of lithium bromide into its constituent ions, resulting in a highly soluble compound. The high solubility of lithium bromide makes it desirable for various applications, including as a desiccant in air conditioning and refrigeration systems, as well as in some chemical reactions and pharmaceutical preparations. 2
Corrosive properties
Lithium bromide is considered corrosive in nature. It can cause corrosion or damage to certain materials and metals, especially when in contact with moisture. When lithium bromide comes into contact with water or humid environments, it undergoes hydrolysis, which produces hydrobromic acid (HBr) as a byproduct. Hydrobromic acid is highly corrosive and can attack metals, particularly those that are susceptible to acid corrosion. In applications where lithium bromide is used as a desiccant in air conditioning and refrigeration systems, precautions are taken to prevent direct contact between the solution and sensitive components. Special materials resistant to corrosion, such as stainless steel and corrosion-resistant alloys, are used for handling and containing lithium bromide solutions. It is important to handle lithium bromide with care, following proper safety procedures and guidelines to avoid personal injury and equipment damage due to its corrosive properties. 3
Safety
Lithium bromide poses potential health risks and requires careful handling to ensure safety. Overexposure to lithium compounds, including lithium bromide, can have severe effects on the central nervous system, resulting in symptoms such as ringing in the ears, vomiting, nausea, blurred vision, diarrhea, drowsiness, and twitching. Inhaling lithium bromide mist can irritate the respiratory organs and may lead to symptoms similar to other lithium compounds, as well as skin rashes resembling acne. Direct contact with lithium bromide can cause skin sensitization, stinging, burning, reddening, dermatitis, and potential allergic reactions. Additionally, it irritates the eyes, causing pain, redness, and potential long-term harm. To mitigate these risks, it is important to handle lithium bromide with caution, using proper ventilation and personal protective equipment (such as gloves, goggles, and respiratory protection) to minimize skin and eye contact. Adhering to local guidelines, regulations, and safety data sheets specific to lithium bromide is crucial for safe handling and use. 4
Reference
1. Wang Z , Orejon D , Sefiane K , Takata Y . Water vapor uptake into hygroscopic lithium bromide desiccant droplets: mechanisms of droplet growth and spreading. Phys Chem Chem Phys, 2019, 21(3):1046-1058.
2. Fu X, Zhang F, Dong C, Zhu W, Xiong K, Pang Z. Efficient homogeneous TEMPO-mediated oxidation of cellulose in lithium bromide hydrates. Int J Biol Macromol, 2021, 191:637-645.
3. Muñoz-Portero MJ, García-Antón J, Guiñón JL, et al. Analysis of Cu corrosion product in aqueous lithium bromide concentrated solutions. Passivation of Metals and Semiconductors, and Properties of Thin Oxide Layers, 2006, 131-136.
4. Lithium Bromide (LiBr): Structure, Properties, Interesting Facts. 2021. https://www.turito.com/blog/chemistry/lithium-bromide.
Related articles And Qustion
See also
Lastest Price from Lithium bromide manufacturers

US $0.10/KG2025-09-14
- CAS:
- 7550-35-8
- Min. Order:
- 1KG
- Purity:
- 99.9%+
- Supply Ability:
- 2000 kgs

US $10.00/KG2025-04-21
- CAS:
- 7550-35-8
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt