Lithium bromide: Structure, Preparation and Application
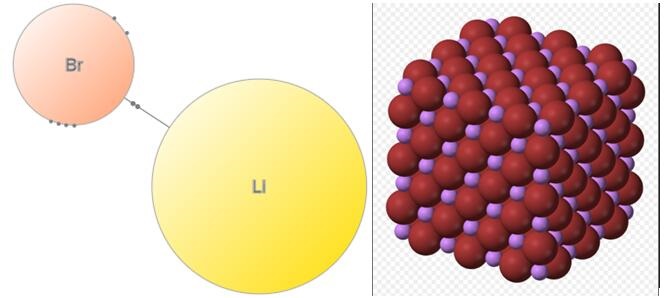
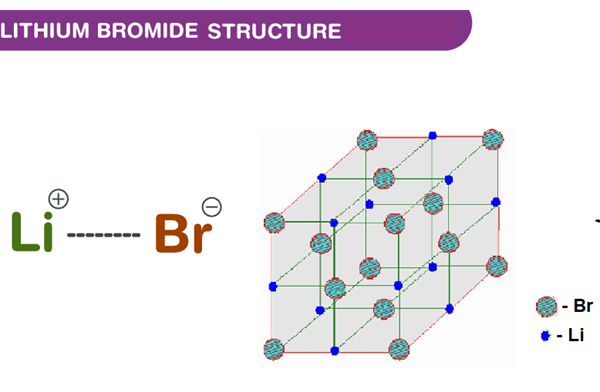
Structure of Lithium bromide
Lithium bromide is an ionic compound that does not share electrons. In lithium bromide, lithium transfers an electron to bromine, forming an ionic bond. As the lithium provides an electron, it forms a cation or positively charged Li+. The bromine, on the other hand, accepts an electron, forming an anion or negatively charged Br-. The crystal structure of lithium bromide is cubic.
Preparation of Lithium bromide
Lithium bromide can be prepared by a number of methods. Several of these methods are listed below:
(1) Lithium bromide can be prepared by reacting lithium carbonate with hydrobromic acid in the formula shown below:
Li2CO3 + 2HBr → 2LiBr + H2CO3
(2) Lithium bromide can also be prepared by reacting lithium hydroxide with hydrogen bromide or aqueous hydrobromic acid, as shown in the reaction formula below:
LiOH + HBr → LiBr + H2O
Application of Lithium bromide
Lithium bromide is a versatile inorganic ionic compound that can be used in several applications:
(1) As a desiccant for air-conditioning machines.
(2) To treat bipolar disorder.
(3) For the synthesis of many organic or inorganic compounds, and for the preparation of drugs.
(4) As an efficient catalyst for imine hydroboration reactions.
(5) Being soluble in ether and other organic solvents, it is used in emulsions.
(6) It is used for the production of lithium hydroxide by recovering lithium from spent lithium bromide.
(7) It is used for heat capacity study of solvent system.
References:
[1] KIM H, KIM H T, LEE J H, et al. Lithium bromide: an inexpensive and efficient catalyst for imine hydroboration with pinacolborane at room temperature†[J]. RSC Advances, 2020. DOI:10.1039/D0RA06023B.
[2] WENJIE GAO. Recycling Lithium from Waste Lithium Bromide to Produce Lithium Hydroxide.[J]. Membranes, 2021. DOI:10.3390/membranes11100759.
[3] S. IYOKI T. U. Heat capacity of the water-lithium bromide system and the water-lithium bromide-zinc bromide-lithium chloride system at high temperatures[J]. International Journal of Refrigeration-revue Internationale Du Froid, 1989. DOI:10.1016/0140-7007(89)90063-7.
Related articles And Qustion
See also
Lastest Price from Lithium bromide manufacturers

US $0.10/KG2025-09-14
- CAS:
- 7550-35-8
- Min. Order:
- 1KG
- Purity:
- 99.9%+
- Supply Ability:
- 2000 kgs

US $10.00/KG2025-04-21
- CAS:
- 7550-35-8
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt