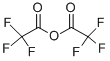
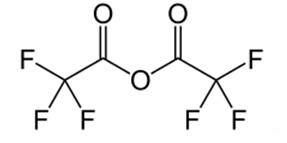
Trifluoroacetic anhydride: Properties and applications
General description
Trifluoroacetic anhydride is a strong electron acceptor, which is often mixed with sodium iodide to form the acceptor reagent. Trifluoroacetic anhydride-sodium iodide mixture (Trifluoroacetic anhydride-I) reacts with several compounds bearing semipolar X-O bond, usually in the mode of deoxygenation, with liberation of iodine. The results of systematic investigations of the Trifluoroacetic anhydride-I-reaction with various types of S-oxo and N-oxo compounds were discussed. The majority of these reactions are characterized by quantitative course, for both X-O substrate transformation as well as the amounts of liberated iodine. On this basis, a variety of sulphoxides and sulphimides were successfully reduced to sulfides and/or arene-N-oxides to amines, using Trifluoroacetic anhydride-I at ambient temperature. Arene- and alkyl-sulphonic acids were reduced to the corresponding thiol derivatives in moderate yields. The Stoichiometric course of the Trifluoroacetic anhydride-I reaction with several S-oxo and N-oxo compounds gave origin to their analytical determinations. Thus, due to stoichiometric amounts of iodine formed from sulphoxides, sulphimides, arene-N-oxides, nitrones, nitroxide radicals, and nitroso compounds - the Trifluoroacetic anhydride-I reagent can be applied to their analytical determination (titrimetric on μmol and spectrometric on the nmol scale) and/or TLC detection. Its appearance is as follows:

Figure 1 Appearance of Trifluoroacetic anhydride
Properties
Trifluoroacetic anhydride, unlike Ac2O, is a weakly associated substance. However, regardless of the fact that this value is close to d values for the lowest alkanes, trifluoroacetic anhydride does not mix with the latter. At the same time, Trifluoroacetic anhydride and Ac2O, while having appreciably different d values, completely mix with each other; moreover, this process is exothermic, and the highest heat effect is (2.17 kJ/mol) is observed at a 1:1 component ratio [1], which have been explained by the possibility of formation of an [oxygen bridge] between electron excessive oxygen atoms in Ac2O and electron-deficient oxygen atoms in Trifluoroacetic anhydride. Thus, Trifluoroacetic anhydride should exhibit electron-acceptor properties. Further evidence for this assumption is also provided by the exothermicity of mixing and the negative pseudo-molar volume DV for the system Trifluoroacetic anhydride [2]. Here, too, the maximum deviation of the property of the system from additivity is observed at a 1:1 component ratio, but the proposed structure of the complex, where one acetone oxygen atom is bound with two oxygen atoms in Trifluoroacetic anhydride, casts some doubt, at least by steric reasons. It is more probable that Trifluoroacetic anhydride forms a donor-acceptor bond with two molecules of the donor, the electron-donor center which is the carbonyl carbon rather than oxygen.
Applications
The trifluoroacetic anhydride - sodium iodide (Trifluoroacetic anhydride-I) reagent, due to solubility of sodium iodide - the most common iodide donor, was the most frequently applied in anhydrous acetone solutions. In such conditions, trifluoroacetic anhydride reacted in two-stage mode with containing semipolar X-O bond compounds, via the O-acylation of X-O bond by means of trifluoroacetic anhydride with formation of the O-acyloxy salts. In the subsequent stage, the salts IA reacted with iodide anion to form intermediary forms IB/IC, which in reaction with second iodide anion afforded the final products ID with simultaneous release of iodine. In addition, trifluoroacetic anhydride was used in preparative deoxygenation of sulphoxides and sulphimides [3], quinoxaline N-oxides, and sulphonic acids and their derivatives [4]. The “analytically useful” character of the reactions of Trifluoroacetic anhydride-I with several containing X-O semipolar bond compounds, namely stoichiometric iodine evolution, short reaction time and very mild conditions, allows the application of this reagent for their quantitative determination and detection. The reaction of Trifluoroacetic anhydride-I with sulphoxides and sulphimides present quantitative character both in respect to conversion to sulphides as well as to quantity of the iodine release. The trifluoroacetic anhydride can be used in the reduction of sulphonic acids and derivatives. The results of reduction of sulphonic acids by TFAA-I show that yields are only moderate.
References
[1]Kreglewski, A. and Wojcicki, W., Bull. Acad. Sci. Pol., Ser. Chim., 1963, vol. 11, no. 11, pp. 6453650.
[2]Kreglewski, A., Bull. Acad. Sci. Pol., Ser. Chim., 1963, vol. 11, no. 5, pp. 3013305.
[3]Drabowicz, J.; ?yz?wa, P.; Miko?ajczyk, M. Synthesis 1981, 890.
[4]Numata, T.; Anano, H.; Oae, S. Tetrahedron Lett. 1980, 21, 1235.
You may like
Related articles And Qustion
See also
Lastest Price from Trifluoroacetic anhydride manufacturers

US $10.00/KG2025-06-10
- CAS:
- 407-25-0
- Min. Order:
- 1KG
- Purity:
- 99.7%
- Supply Ability:
- 10 ton

US $19.90/kg2025-04-21
- CAS:
- 407-25-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt