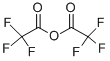
Trifluoroacetic anhydride- an important organic reagent
Trifluoroacetic anhydride (TFAA) is a colorless liquid with a vinegar odor and a volatile color. It has strong acidity and trifluoromethyl group. The molecular formula of TFAA is C4F6O3. The molecular weight is 210.02. The boiling point is 40°C. The melting point is -65°C. The density is 1.49 g/cm3. The vapor pressure is 6 psi (20°C). TFAA violently reacts with water to form water soluble trifluoroacetic acid. It is corrosive and moisture sensitive. It is toxic by inhalation. It should be freshly distilled prior to reaction and used in a fume hood.
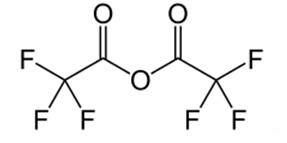
Trifluoroacetic anhydride is the acid anhydride of trifluoroacetic acid. It is the perfluorinated derivative of acetic anhydride. Like many acid anhydrides, it may be used to introduce the corresponding trifluoroacetyl group. The corresponding acyl chloride, trifluoroacetyl chloride, is a gas, making it inconvenient to work with. Trifluoroacetic anhydride is the recommended desiccant for trifluoroacetic acid [1].
Trifluoroacetic anhydride can be prepared from trifluoroacetic acid by dehydrating with excess α-halogenated acid chlorides. For example, with dichloroacetyl chloride [2]:
2CF3COOH + Cl2CHCOCl→(CF3CO)2O + Cl2CHCOOH + HCI
Trifluoroacetic anhydride is an acylation reagent and protection reagent. TFAA is reactive towards amines, alcohols and phenol functional groups. As a gas chromatography (GC) derivatization reagent, TFAA derivatives are often introduced for the sake of improving gas chromatographic resolution and improved detection with ECD detectors.
The reaction between trifluoroacetic acid anhydride (TFAA) and compounds containing amines or alcohol moieties is facile and the reaction is easy to conduct. After the preparation of O-trifluoroacetyl and N-trifluoroacetyl derivatives, gas chromatograms tend to be significantly improved due to decreased analyte polarity and increased sample volatility.
Trifluoroacetic anhydride provides a convenient way to introduce a trifluoromethyl group into an organic compound. It is used in the production of agricultural and pharmaceutical molecules. It is also used heavily in chromatography. TFAA is the most volatile and reactive of the anhydrides and it reacts with alcohols, amines, and phenols. TFAA can be used in organic synthesis, nuclear magnetic resonance (NMR) solvent, gas chromatography, liquid chromatography, etc.
TFAA is suitable for:
• Used as catalyst for a wide range of reactions including esterifications, condensations, and oxidations.
• Esterification and transesterification promoter.
• Promotes condensation reactions involving acyl groups and aromatic or unsaturated compounds.
• Solvent for chromatography.
When reacted with a compound containing an active hydrogen it will yield a trifluoromethyl compound and trifluoroacetic acid as byproduct. It is an important agent in organic synthesis and is used in trifluoacylation reactions like o-acylation, n-acylation or c-trifluoro-acylation.
TFAA reacts violently with water and alkaline metals. Contact with the skin produces TFA and causes deep burns in a short time. To treat an accident, flush continuously with a tremendous excess of water as quickly as possible. Instant action will minimize the penetration and extent of injury but probably not stop some effects of a burn. Cover the area with sterile gauze and obtain medical treatment. Inhalation of acid fumes produces irritation typical of any strong acid. Ventilation or an air mask are recommended for any appreciable concentration.
TFAA is an important reagent for the production of a novel class of bio-based surfactants. Current manufacturing methods using trifluoroacetic acid (TFA acid) have been completed on a lab-scale and involve the use of halogens or phosphorous pentoxide; there does not yet exist a continuous method for TFAA synthesis from TFA acid without the aforementioned hazardous compounds. In 2018, Clare Wunder has proposed a two-step process for recovering TFA acid from the production of bio-based surfactants, then synthesizing TFAA through reactive distillation of TFA acid and acetic anhydride. First, it was found that TFA acid can be continuously distilled from the bio-based molecule precursor solution. In this step the heat applied for distillation drives the reaction to near completion, so reagents can be used in a 1:1:1 ratio to minimize waste. 95% of the TFA acid was recovered in a heterogeneous mixture with the heptane solvent, so it can be decanted and purified by distillation for use in TFAA synthesis. Second, it was found that reactive distillation of a stoichiometric ratio of TFA acid and acetic anhydride (2:1) produced TFA anhydride with 94% purity and conversion of 9.0%. Finally, experimental results were used to construct a flowsheet in Aspen Plus, but the heterogeneous azeotropic nature of the first step prevented the use of simulation for predicting results after scale-up. Due to this complication and the low conversion of TFA acid to TFA anhydride, this process will require further investigation before it can be recommended for large-scale production.
References
[1] Chai, Christina Li Lin; Armarego, W. L. F. (2003). Purification of laboratory chemicals (Google Books excerpt). Oxford: Butterworth-Heinemann. p. 376. ISBN 0-7506-7571-3.
[2] US 4595541
[3] https://en.wikipedia.org/wiki/Trifluoroacetic_anhydride
You may like
Related articles And Qustion
See also
Lastest Price from Trifluoroacetic anhydride manufacturers

US $10.00/KG2025-06-10
- CAS:
- 407-25-0
- Min. Order:
- 1KG
- Purity:
- 99.7%
- Supply Ability:
- 10 ton

US $19.90/kg2025-04-21
- CAS:
- 407-25-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt