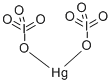
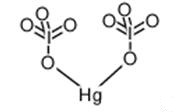
Electronic and Optical Properties of Mercuric Iodate
Mercury(II) iodate(V) is dimorphous and crystallizes in two polymorphs named α- and β-Hg(IO3)2. The α-modification was prepared by precipitation of a slightly acidified Hg(NO3)2 solution with an excess of an aqueous HIO 3 solution; for the polycrystalline material unindexed powder data is given. Colourless to light-yellow single crystals of the β-modification, with an edge-length of up to 0.3 mm and a plate-like habit, were grown during hydrothermal treatment (220°C, 10 d) of the polycrystalline precipitate. The crystal structure of β-Hg(IO3)2 was determined from single crystal X-ray data (space group P21 (no. 4), Z = 2, a = 5.7818(9), b = 5.6077(10), c = 8.9849(12) A, β = 102.890(2)°C, 1668 structure factors, 83 parameter, R[F2 > 2σ(F 2)] = 0.0175, wR(F2 all) = 0.0382) and is made up from trigonal pyramidal IO3 groups with an average I-O distance of 1.825 A and distorted [HgO8] polyhedra with a mean Hg-O bond length of 2.537 A as the main building units. Infinite zig-zag chains of cis-corner-sharing [HGO4/2O4/1] polyhedra extend parallel to [010]. The chains are connected by IO3 groups along the [100] direction to form layers parallel to the (001) plane. The three-dimensional framework is held together by weak intermolecular I-O interactions > 2.58 A along the [001] direction. No α ↔ β phase transformation was detected upon heating. Both polymorphs decompose completely in an one-step mechanism around 500°C.[1]
It is shown that the mercury-mercuric iodate electrode is reproducible and reversible, provided that measurements are made before the spontaneous reaction between the electrode components has produced significant amounts of mercurous iodate. In addition, it is found that the entropy of mercuric iodate is so small that the reaction Hg(1) plus Hg(IO//3)//2(s) equals Hg//2(IO//3)//2(s) proceeds with an entropy increase of 33 J multiplied by (times) mole-1 minus-1 multiplied by (times) K-1 minus-1.[2]
Full-potential linearized augmented plane wave calculations were performed for the Hg(IO3)2 compound in order to investigate the structural, electronic and optical properties. The calculated lattice constants, bulk modulus and first order pressure derivative of the bulk modulus are reported. The electronic structure indicate that Hg(IO3)2 has a direct band gap of 2.61 eV. The imaginary part of the dielectric functions is calculated and the contributions of various transitions peaks were analyzed. Furthermore, the other optical properties have been investigated.[3]
The noncentrosymmetric material Hg(IO3)2 with P21 space group symmetry has been synthesised. This compound is thermally very stable (520 °C) and shows a NLO efficiency visually comparable to that of lithium iodate. The crystal structure has been solved in order to relate this efficiency to the IO3 chomophore packing. Simple calculations, based on the bond additivity model, show that both I-O and Hg-O bonds contribute to SHG tensor.[4]
References
1. Weil M. Dimorphism in mercury(II) iodate(V): Preparation and thermal behaviour of α- and β- Hg(IO3)2, and single crystal structure analysis of β- Hg(IO3)2[J]. Zeitschrift fur Naturforschung - Section B Journal of Chemical Sciences, 2003, 58(7):627 – 632.
2. Nash. Standard Potential of the Mercury-Mercuric Iodate Electrode[J]. Journal of the Electrochemical Society, 1978, 125(6):875 - 877
3. Lagoun B, Bentria B, Lefkaier IK. Ab initio calculation of structural, electronic and optical properties of Hg(IO3)2[J]. Physica B: Condensed Matter, 2014, 433(1):117 - 121
4. Bentria B. Benbertal D, Bagieu-Beucher M. Mosset A, Zaccaro J, Crystal engineering strategy for quadratic nonlinear optics. Part II: Hg(IO3)2[J]. Solid State Sciences, 2003, 5(2):359 - 365