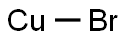Applications of Cuprous bromide
Cuprous bromide is the chemical compound with the formula CuBr. This diamagnetic solid adopts a polymeric structure akin to that for zinc sulfide[1]. Cuprous bromide is widely used in the synthesis of organic compounds and as a lasing medium in copper bromide lasers. Cuprous bromide may be prepared by reduction of cupric bromide or CuSO4-NaBr.In molten state; Cuprous bromide is grayish brown /greenish brown in color[2]. Cuprous Bromide is also known as Bromocopper, Copper Monobromide, Copper I Bromide, Cuprous Bromide, 7787-70-4, AG-H-12192, Copper bromide (CuBr), AC1L2NJJ, AC1Q23SS and comes with Molecular Formula of BrCu and Molecular Weight of 143.45.
Cuprous bromide is manufactured through reduction of copper(II) sulfate solution which contains potassium bromide with metallic copper/sulfur dioxide/other standard reducing agents. Cuprous bromide can be made available in different forms like in form of white tetrahedral crystals or white powder or cubic crystals (zinc blende structure) or as white crystalline solid that changes to green in light. Its properties include Boiling Point of 1345°C, Melting Point of 504°C, Density/Specific Gravity of 4.72@25°C/4°C and solubility in cold water; ammonium hydroxide, hydrochloric acid and hydrobromic acid. Cuprous bromide is a copper(I) halide. Cuprous bromide bromide along with N-(n-octyl)-2-pyridylmethanimine participates in the living-radical polymerization of methyl methacrylate. Cuprous bromide reacts with various heterocyclic thiones (L) in the presence of triphenylphosphine, to afford the mononuclear complexes of the general formula [Cu(L)(PPh3)2Br].

Powder of Cuprous bromide
The compound is white, although samples are often colored due to the presence of copper(II) impurities. The copper(I) ion also oxidizes easily in air. It is commonly prepared by the reduction of cupric salts with sulfite in the presence of bromide. For example, the reduction of copper(II) bromide with sulfite yields copper(I) bromide and hydrogen bromide[3,4]:
2 CuBr2 + H2O + SO32- → 2 CuBr + SO42- + 2 HBr
CuBr is insoluble in most solvents due to its polymeric structure, which features four-coordinated, tetrahedral Cu centers interconnected by bromide ligands (ZnS structure). Upon treatment with Lewis bases, CuBr converts to molecular adducts. For example with dimethyl sulfide, the colorless complex is formed[5]:
CuBr + S(CH3)2 → CuBr(S(CH3)2)
In the Sandmeyer reaction, CuBr is employed to convert diazonium salts into the corresponding aryl bromides:
ArN2 + CuBr → ArBr + N2 + Cu
References
[1] Budavari, S. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996., p. 448.
[2] Zhang Y C , Tang J Y , Zhou W D . Green hydrothermal synthesis and optical properties of cuprous bromide nanocrystals[J]. Materials Chemistry & Physics, 2008, 108(1):4-7.
[3] Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001.
[4] This report gives a procedure for generating CuBr: Jonathan L. Hartwell (1955).
[5] Jarowicki, K.; Kocienski, P. J.; Qun, L. "1,2-Metallate Rearrangement: (Z)-4-(2-Propenyl)-3-Octen-1-ol" Organic Syntheses, Collected Volume 10, p.662 (2004)
You may like
Related articles And Qustion
Lastest Price from Cuprous bromide manufacturers

US $100.00-80.00/KG2025-07-08
- CAS:
- 7787-70-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000000

US $10.00/KG2025-04-21
- CAS:
- 7787-70-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt