Applications of Benzeneacetonitrile
Benzeneacetonitrile (abbreviated BnCN) is a colorless, oily liquid with an aromatic odor. Benzeneacetonitrile is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry[1].
Benzyl cyanide can be hydrolyzed to give phenylacetic acid or used in the Pinner reaction to yield phenylacetic acid esters. The compound also forms an "active methylene unit" on the carbon between the aromatic ring and the nitrile functional group. This active carbon, referred to as a nitrile anion, is a useful reactive intermediate for the formation of new carbon-carbon bonds[2-4].
Benzeneacetonitrile is synthesized by reaction of benzyl chloride with potassium cyanide or sodium cyanide . Benzeneacetonitrile is a natural constituent of plants and is a constituent of foods, particularly citrus fruits, papaya, cabbage, mushrooms, roasted onions, tomatoes, cocoa, tea, roasted peanuts and cauliflower . Benzeneacetonitrile, at least in part, is formed by breakdown of benzylglucosinolate in the plant material. Benzeneacetonitrile also is found in tap water, river water, sewage and in cigarette smoke ).
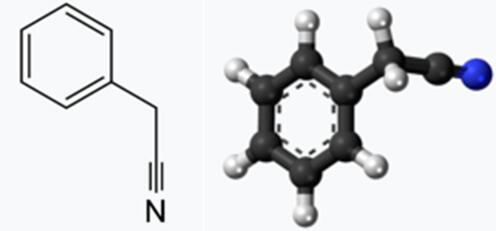
Fig 1. Chemical structure formula and three-dimensional structure of Benzeneacetonitrile
Preparation: 6 kilos of potassium cyanide are dissolved in water in an enameled stirring vessel fitted with reflux condenser. There is then added slowly a solution of 10 kilos of benzyl chloride[5] in an equal weight of alcohol. When all has been added, the mixture is heated for three to four hours at the boil. The liquid separates into two layers, the upper one reddish brown, contains the Benzeneacetonitrile, the lower aqueous layer the alcohol and potassium chloride (which is precipitated as a crust on the walls of the vessel on cooling). The brown oily layer is washed several times with water and then distilled at ordinary pressure. Traces of alcohol first come over and the temperature then rises quickly to 195ºC. The residual liquor is now cooled somewhat and the Benzeneacetonitrile distilled off in vacuo without fractionation. The crude Benzeneacetonitrile so obtained is used for the next operation.
References
[1] omenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 16.
[2] Makosza, M.; Jonczyk, A (1976). "Phase-Transfer Alkylation of Nitriles: 2-Phenylbutyronitrile". Organic Syntheses. 55: 91.
[3] Itoh, Masumi; Hagiwara, Daijiro; Kamiya, Takashi (1988). "New Reagent for tert-Butoxycarbonylation: 2-tert-Butoxycarbonyloxyimino-2-phenylacetonitrile". Organic Syntheses. 6: 199.
[4] Wawzonek, Stanley; Smolin, Edwin M. (1955). "α-Phenylcinnamonitrile". Organic Syntheses. 3: 715.
[5] J F Norris, American Chemical Journal 38: 627-642 (1907).