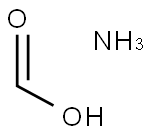
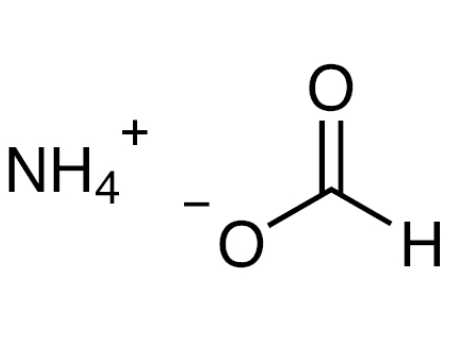
Applications of Ammonium Formate
Ammonium formate, NH4HCO2, is the ammonium salt of formic acid. Ammonium formate is a colorless, hygroscopic, crystalline solid. Ammonium formate is a white solid with a weak odor of ammonia. Sinks and mixes slowly with water. If wet ammonium formate is left to dry in a desiccator over the course of weeks or months, tiny white crystals of dry ammonium formate shaped like snowflake arms or fern leaves will form on the edge of the flask.
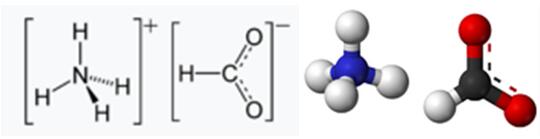
Fig 1. Chemical structure formula and three-dimensional structure of Ammonium formate
Pure ammonium formate decomposes into formamide and water when heated, and this is its primary use in industry. Formic acid can also be obtained by reacting ammonium formate with a dilute acid, and since ammonium formate is also produced from formic acid, it can serve as a way of storing formic acid.
Ammonium formate can also be used in palladium on carbon (Pd/C) reduction of functional groups. In the presence of Pd/C, ammonium formate decomposes to hydrogen, carbon dioxide, and ammonia. This hydrogen gas is adsorbed onto the surface of the palladium metal, where it can react with various functional groups. For example, alkenes can be reduced to alkanes, or formaldehyde to methanol. Activated single bonds to heteroatoms can also be replaced by hydrogens (hydrogenolysis)[1].
Ammonium formate can be used as a buffer in high performance liquid chromatography (HPLC), and is suitable for use with liquid chromatography-mass spectrometry (LC/MS). The pKa values of formic acid and the ammonium ion are 3.8 and 9.2, respectively[2].
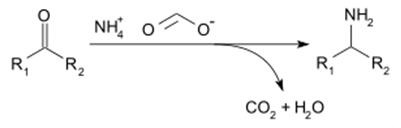
Ammonium formate can be used for reductive amination of aldehydes and ketones (Leuckart reaction), by the following reaction[3]:
When heated, ammonium formate eliminates water, forming formamide. Upon further heating, Ammonium formate forms hydrogen cyanide (HCN) and water. A side reaction of this is the decomposition of formamide to carbon monoxide (CO) and ammonia.
Preparation: Ammonium formate can be made by bubbling ammonia through formic acid, though this requires lots of ammonia. An ammonium salt, such as ammonium bicarbonate can be used instead. Cooling the solution will cause the salt to precipitate. Excess water can be evaporated by carefully heating the solution, at below 115℃, to prevent it from melting/decomposing. Filter the resulting precipitate and leave it to dry, either in open air or in a desiccator. Heating will not dry ammonium formate, instead it will decompose, resulting in a yellow syrup containing water, formamide and ammonium formate[4].
Ammonium formate should be kept in closed bottles, away from moisture. Can be stored in a desiccator. Ammonia may be added to limit hydrolysis.
Ammonium formate may release formic acid vapors and protection should be worn when handling the compound.
References
[1] Z. Dobrovolná, L. Červený. Ammonium formate decomposition using palladium catalyst[J]. Research on Chemical Intermediates, 2000, 26(5):489-497.
[2] Draper, William M, Xu, Dadong, Perera, S. Kusum. Electrolyte-Induced Ionization Suppression and Microcystin Toxins: Ammonium Formate Suppresses Sodium Replacement Ions and Enhances Protiated and Ammoniated Ions for Improved Specificity in Quantitative LC-MS-MS[J]. Analytical Chemistry, 81(10):4153-4160.
[3] Alexander, Elliot; Ruth Bowman Wildman (1948). "Studies on the Mechanism of the Leuckart Reaction". Journal of the American Chemical Society. 70: 1187–1189.
[4] J. Tsuji, T. Sugiura, I. Minami. Preparation of 1,2-Dienes by the Palladium-Catalyzed Hydrogenolysis of 3-Methoxycarbonyloxy-1-alkynes with Ammonium Formate[J]. Cheminform, 1987(7):603-606.
You may like
Related articles And Qustion
See also
Lastest Price from Ammonium formate manufacturers

US $0.00-0.00/kg2025-09-23
- CAS:
- 540-69-2
- Min. Order:
- 25kg
- Purity:
- 98%min
- Supply Ability:
- 20 TONS

US $1200.00-1100.00/ton2025-08-05
- CAS:
- 540-69-2
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M