Applications of 4,4'-Methylenedianiline
4,4'-Methylenedianiline (MDA) is an organic compound with the formula CH2(C6H4NH2)2. 4,4'-Methylenedianiline is a colorless or white solid. 4,4'-Methylenedianiline is produced on industrial scale as a precursor to polyurethanes. 4,4'-Methylenedianiline appears as a tan flake or lump solid with a faint fishlike odor. 4,4'-Methylenedianiline may be toxic by inhalation or ingestion, and may be irritating to skin. Insoluble in water. 4,4'-Methylenedianiline is an aromatic amine that is diphenylmethane substituted at the 4-position of each benzene ring by an amino group. 4,4'-Methylenedianiline has a role as a carcinogenic agent. 4,4'-Methylenedianiline derives from a hydride of a diphenylmethane.
4, 4'-methylenedianiline belongs to the family of Diphenylmethanes. These are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups.
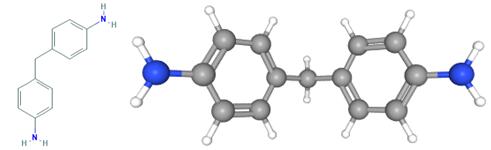
Fig 1. Chemical structure formula and three-dimensional structure of 4,4'-Methylenedianiline
In the industrial production, 4,4'-Methylenedianiline is produced by reaction of formaldehyde and aniline in the presence of hydrochloric acid. This reaction consumes the majority of aniline produced worldwide.
4,4'-Methylenedianiline is consumed mainly as a precursor to 4,4 ́-methylene diphenyl diisocyanate (MDI). 4,4'-Methylenedianiline is treated with phosgene to give MDI. MDI is a precursor to many polyurethane foams. Lower quantities are used as hardeners in epoxy resins and adhesives, as well as in the production of high-performance polymers. 4,4'-Methylenedianiline is hydrogenated to give 4,4-diaminodicyclohexylmethane, which is also used in polymer chemistry[1-4].
4,4'-Methylenedianiline polymerizes if heated above 257℃. Incompatible with strong oxidizing agents. 4,4'-Methylenedianiline is also incompatible with acids. Catalyzes isocyanate-alcohol and epoxide reactions. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
4,4'-Methylenedianiline is considered a potential occupational carcinogen by the US National Institute for Occupational Safety and Health. The Occupational Safety and Health Administration has set a permissible exposure limit at 0.01 ppm over an eight-hour time-weighted average, and a short-term exposure limit at 0.10 ppm[5].
4,4′-Methylenedianiline (MDA) is a primary aromatic amine used in the plastics industry and is classified by the International Agency for Research on Cancer as an animal carcinogen and possible human carcinogen. In order to estimate human exposure it is useful to determine percutaneous penetration. Previous studies have suggested that both rat and human skin were permeable to MDA, with greater penetration being seen through human skin[6].
References
[1] Christian Six, Frank Richter (2005). "Isocyanates, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
[2] ToxFAQs for 4,4'-Methylenedianiline, Agency for Toxic Substances and Disease Registry.
[3] Karsten Eller; Erhard Henkes; Roland Rossbacher; Hartmut Höke (2005). "Amines, Aliphatic". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
[4] Data on manufacture, import, export, uses and release of 4-4’ diaminodiphenylmethane as well as Archived 2011-10-01 at the Wayback Machine.
[5] Kopelman, H; Robertson, MH; Sanders, PG; Ash, I. "The Epping jaundice". Br Med J. 1: 514–6.
You may like
Related articles And Qustion
See also
Lastest Price from 4,4'-Methylenedianiline manufacturers

US $10.00/KG2025-04-21
- CAS:
- 101-77-9
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $8.00/kg2024-11-07
- CAS:
- 101-77-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20000