Tosylmethyl isocyanide: Reaction profile and Toxicity
Introduction
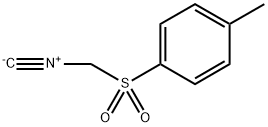
4-tolylsulfonylmethyl isocyanide is also known as TosMIC or tosylmethyl isocyanide. TosMIC is a multipurpose synthetic reagent. It is by far the most versatile synthon derived from methyl isocyanide. TosMIC is the only commercially available sulfonylmethyl isocyanide[1]. It is a stable, colourless, practically odourless solid which can be stored at room temperature without decomposition.
Reaction profile
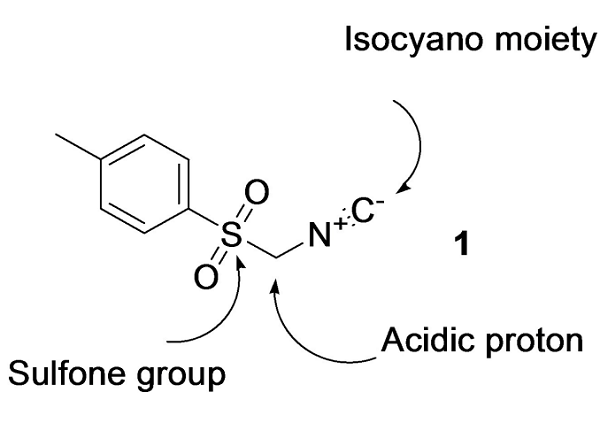
Tosylmethyl isocyanide, also known as van Leusen's reagent. It contains three densely functionalized groups, namely the isocyanide and sulfonyl functionality, and an alpha carbon has an acidic nature between them. The sulfonyl group serves as a good leaving group, augmenting the strength of acidity of the α-carbon. TosMIC is also reported for unanticipated synthesis of sulfones and sulfinates. It has also been proved that TosMIC is involved in chemo, regio and stereoselective synthesis without disturbing the selectivity. Mono- and disubstitutions at the α-position derive the sundry functionalized molecules[2]. TosMIC was introduced and extensively applied in organic synthesis by the Dutch professor van Leusen. Due to the establishment of the applicability of TosMIC in heterocyclic synthesis by van Leusen, it is now recognized as van Leusen's reagent 1. TosMIC is very reactive due to less steric hindrance and increased electrophilic nature.
Toxicity
Causes respiratory tract irritation. Irritant. Harmful if swallowed, inhaled, or absorbed through the skin. Causes eye and skin irritation. It may cause digestive tract irritation. It may cause central nervous system depression. May cause cardiac disturbances. Metabolized to cyanide in the body, which may cause headache, dizziness, weakness, unconsciousness, convulsions, coma and possible death. Moisture sensitive. Target Organs: Central nervous system, cardiovascular system.
References
[1] D. V. Leusen, A. Leusen. “Synthetic Uses of Tosylmethyl Isocyanide (TosMIC).” Organic Reactions 30 1 (2004).
[2] Kumar, Dr. Kapil. “TosMIC: A Powerful Synthon for Cyclization and Sulfonylation.” ChemistrySelect 5 33 (2020): 10298–10328.
You may like
Related articles And Qustion
See also
Lastest Price from Tosylmethyl isocyanide manufacturers

US $1.00/KG2025-04-21
- CAS:
- 36635-61-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 36635-61-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month