Applications of Tosylmethyl isocyanide
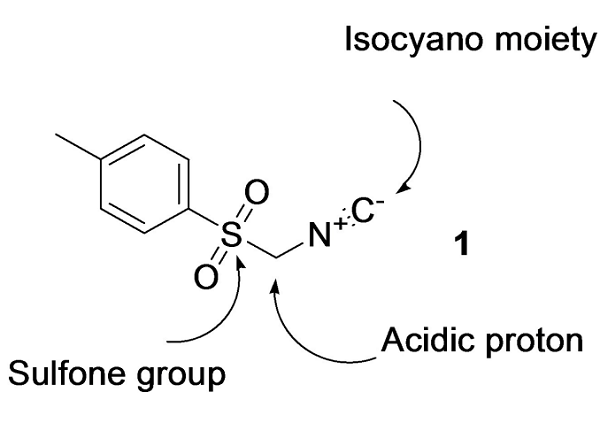
Tosylmethyl isocyanide(TosMIC) is pale yellow to light brown crystalline powder. Tosylmethyl isocyanide for use in the preparation of biologically active pyrroles and imidazoles. Tosylmethyl isocyanide is a unique one-carbon synthon that can be easily deprotonated and alkylated. The sulfinyl group not only enhances the acidity of the α-protons, but Tosylmethyl isocyanide is also a good leaving group. In addition, Tosylmethyl isocyanide features the unique properties of the isocyanide group in which the oxidation of the carbon atom is a driving force for multiple reactions[1].
Tosylmethyl isocyanide is an organic compound with the formula CH3C6H4SO2CH2NC. The molecule contains both sulfonyl and isocyanide groups. It is a colourless solid that, unlike many isocyanides, is odorless. Tosylmethyl isocyanide is prepared by dehydration of the related formamide derivative. Tosylmethyl isocyanide is used in the Van Leusen reaction which is used to convert aldehydes to nitriles or in the preparation of oxazoles and imidazoles[2]. The versatility of Tosylmethyl isocyanide in organic synthesis has been documented[3]. Tosylmethyl isocyanide is a fairly strong carbon acid, with an estimated pKa of 14 (compared to 29 for methyl tolyl sulfone), the isocyano group acting as an electron acceptor of strength comparable to an ester group[4]. Tosylmethyl isocyanide is the only commercially available sulfonylmethyl isocyanide. Tosylmethyl isocyanide is a stable, colorless, practically odorless solid, which can be stored at room temperature without decomposition.
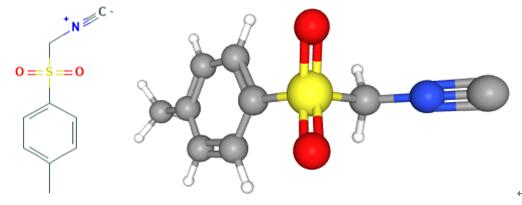
Fig 1. Chemical structure formula and three-dimensional structure of Tosylmethyl isocyanide
Tosylmethyl isocyanide is used as a synthetic reagent in the preparation of variety or biologically active heterocycles such as pyrroles and imidazoles. Tosylmethyl isocyanide is reported to inhibit [ Fe]-hydrogenase with very high affinity.
An unprecedented silver-catalyzed cascade reaction of Tosylmethyl isocyanide with propargylic alcohols for the efficient synthesis of (E)-vinyl sulfones has been developed where Tosylmethyl isocyanide plays a dual role as both the reactant in the allenylation of propargylic alcohols and the sulfonyl source[5].
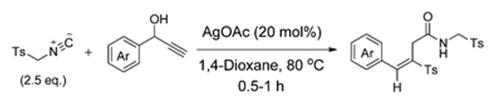
Tosylmethyl isocyanide is a synthetic reagent used in the synthesis of triplet drugs with the 1,3,5-trioxazatriquinane skeleton, pyrroles, oxazoles and in imidazoles. Tosylmethyl isocyanide serves as an isonitrile component in a diastereoselective Passerini reaction. Furthermore, Tosylmethyl isocyanide is used in the Van Leusen reaction to convert aldehydes to nitriles.
References
[1] Chemistry of sulfonylmethyl isocyanides. 13. A general one-step synthesis of nitriles from ketones using tosylmethyl isocyanide. Introduction of a one-carbon unit O. H. Oldenziel, D. Van Leusen, A. M. Van Leusen, J. Org. Chem., 1977, 42, 3114-3118.
[2] Keeri, Abdul Raheem; Gualandi, Andrea; Mazzanti, Andrea; Lewinski, Janusz; Cozzi, Pier Giorgio (2015-12-21). "Me2Zn-Mediated Catalytic Enantio- and Diastereoselective Addition of TosMIC to Ketones". Chemistry – A European Journal. 21 (52): 18949–18952.
[3] Hoogenboom, B. E.; Oldenziel, O. H.; van Leusen, A. M. (1977). "p-TOLYLSULFONYLMETHYL ISOCYANIDE". Organic Syntheses. 57: 102.; Collective Volume, 6, p. 987
[4] van Leusen, Albert M.; van Leusen, Daan; Czakó, Barbara (2008-09-15), "p-Tolylsulfonylmethyl Isocyanide", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd.
[5] Silver-catalyzed cascade reaction of tosylmethyl isocyanide (TosMIC) with propargylic alcohols to (E)-vinyl sulfones: dual roles of TosMIC[J]. Org. Biomol. Chem. 2015, 13(32):8723-8728.
Related articles And Qustion
See also
Lastest Price from Tosylmethyl isocyanide manufacturers

US $1.00/KG2025-04-21
- CAS:
- 36635-61-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 36635-61-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month