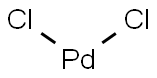Applications of Palladium chloride
Palladium chloride is brown to brownish violet powder, red rhombic crystals; hygroscopic. Palladium chloride is readily soluble in hydrochloric acid and solutions of alkali metal chlorides. When Palladium chloride is heated to decomposition, it emits highly toxic fumes of /hydrogen chloride/, and Palladium chloride decomposed at high temperature to palladium and chlorine[1]. Palladium chloride, is also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. Palladium chloride is prepared by the reaction of chlorine with palladium metal at high temperatures.
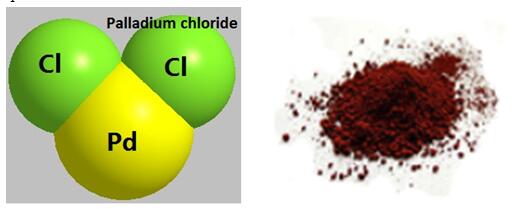
Fig 1. Chemical structure formula and powder of Palladium chloride
Palladium chloride is prepared by dissolving palladium metal in aqua regia or hydrochloric acid in the presence of chlorine. Alternatively, Palladium chloride may be prepared by heating palladium sponge metal with chlorine gas at 500 °C.
The effect of Palladium chloride on the activity of CPK-MM creatine kinase of rabbit's muscles and human serum was examined and a dose-dependent inhibition of the enzymatic activity, accompanied by a considerable increase in the electrophoretic enzyme mobility toward anode, were noted. Analysis of the Pd-CPK-MM bond has shown that the cation forms an extremely stable bond with the enzyme sulfhydryl groups, resulting in defective energy metabolism in the organism[2].
Palladium chloride is used in plating baths. Pellets or monoliths of oxidation catalysts are either immersed in an aqueous solution of palladium chloride (impregnation technique) or sprayed with a solution of this chemical[3]. In photography, for preparing pictures to be transferred to porcelain; toning solutions; electroplating parts of clocks and watches; manufacture of indelible ink; for preparation of the metal for use as a catalyst; palladium dichloride paper is used for detecting carbon monoxide, to find leaks in buried gas pipes. Preparation of palladium catalysts.
Palladium chloride is used to remove the stain present in stainless steel . Its solutions are used to test the corrosion-resistance of stainless steel. Palladium chloride is used in carbon monoxide detectors. Palladium chloride is also used for cosmetic tattooing of leukomas in the cornea and in the preparation of palladium iodide by reacting with potassium iodide. Palladium chloride is involved in the synthesis of semiconducting metal-containing polymers. Palladium chloride is used as a catalyst for carbonylation of organic tellurides and deamination of phenethylamines to phenyl substituted pyrroles with copper(I) as co catalyst. Palladium chloride plays an important role in the hydrodimerization of butadiene to form octa-2,7-dien-1-ol[4,5].
References
[1] O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1252.
[2] Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V3 p.279.
[3] WHO; Environ Health Criteria 226: Palladium (2002) Available from, as of March 3, 2005.
[4] Janreddy, D.; Kavala, V.; Kotipalli, T.; Kuo, C.; Kuo, T.; Chen, M.; He, T.; Yao, C. Palladium(II) Chloride-Catalyzed Aerobic Oxidative Intermolecular Cycloaddition Reaction of 2-Alkynylbenz- aldehydes and Electron-Deficient Terminal Alkenes: An Efficient Synthesis of Naphthyl Ketones. Adv. Synth. Catal. 2014, 356 (14-15), 3083-3091.
[5] Banti, C. N.; Charalampou, D. C.; Kourkoumelis, N.; Owczarzak, A. M.; Kubicki, M.; Hadjikakou, S. K.; Hadjiliadis, N. Mono-nuclear cis-Pd(II) chloride complex of the thio-nucleotide analogue 5-methyl-thiouracil and its biological activity. Polyhedron 2015, 87, 251-258.
You may like
Related articles And Qustion
See also
Lastest Price from Palladium chloride manufacturers

US $2500.00/g2025-04-29
- CAS:
- 7647-10-1
- Min. Order:
- 1g
- Purity:
- 98
- Supply Ability:
- 500 Kg

US $0.00/kg2025-04-25
- CAS:
- 7647-10-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg