Tosylmethyl Isocyanide: Applications in Medicinal Chemistry and its Preparation Method
General Description
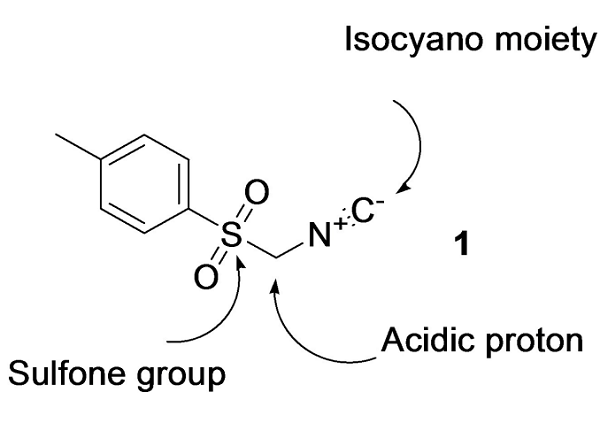
TosMIC is the acronym for 4-tolylsulfonylmethyl isocyanide or tosylmethyl isocyanide. It is the best-known member of a series of about 25 sulfonyl-substituted methyl isocyanides RSO2CH2NC. TosMIC is a multipurpose synthetic reagent. It is by far the most versatile synthon derived from methyl isocyanide. TosMIC is the only commercially available sulfonylmethyl isocyanide. It is a stable, colorless, practically odorless solid, which can be stored at room temperature without decomposition.

Figure 1. Tosylmethyl isocyanide
Applications in Medicinal Chemistry1
Tosylmethyl isocyanide is a versatile reagent in medicinal chemistry, widely recognized for its utility in synthesizing pharmacologically active compounds. One of the critical applications of Tosylmethyl isocyanide involves the development of inhibitors for various diseases, including the formulation of novel HIV-1 attachment inhibitors. By functioning as a pivotal building block, Tosylmethyl isocyanide facilitates the introduction of unique structural motifs essential for binding to biological targets such as the HIV-1 gp120 protein.
The importance of Tosylmethyl isocyanide in medicinal chemistry is exemplified through its role in the synthesis of 4-fluoro-6-azaindole derivatives, which are potent HIV-1 attachment inhibitors. These inhibitors target the gp120 protein, crucial for the virus's attachment and entry into host cells. Tosylmethyl isocyanide aids in constructing the azaindole core, a key component that allows for further functionalization and enhancement of molecular interactions with gp120. The utility of Tosylmethyl isocyanide extends to optimizing the structure-activity relationships (SAR) of these inhibitors.
By facilitating modifications at critical positions of the molecular backbone, Tosylmethyl isocyanide supports the exploration of various substituents that improve the binding affinity and pharmacokinetic properties of the inhibitors. For instance, substitutions facilitated by Tosylmethyl isocyanide in the 7-position of the azaindole core lead to compounds with enhanced potency and desirable in vivo profiles. Furthermore, the ability of Tosylmethyl isocyanide to introduce substituents that can adopt a coplanar conformation with the azaindole core is vital. This structural arrangement, supported by Tosylmethyl isocyanide, is crucial for the efficacy of HIV-1 inhibitors as it ensures optimal alignment and interaction with the target protein. therapeutic agents.
Preparation Method2
The synthesis of Tosylmethyl isocyanide involves a two-step reaction starting with sodium p-toluenesulfinate as the primary material. Initially, sodium p-toluenesulfinate undergoes a reaction reminiscent of the Mannich reaction, which is a critical step in forming the intermediate compound necessary for the subsequent transformation into Tosylmethyl isocyanide. This step is crucial as it sets the foundation for the isocyanide group formation. Following the initial Mannich-like reaction, a hydrolysis step is employed. This step is essential for converting the intermediate into Tosylmethyl isocyanide by introducing the isocyanide functional group, which is a defining characteristic of Tosylmethyl isocyanide. The efficiency of this hydrolysis reaction is instrumental in defining the overall yield and purity of the final product. The overall yield of Tosylmethyl isocyanide reaches 72.3% based on the initial amount of sodium p-toluenesulfinate used, under optimized reaction conditions. This high yield highlights the efficiency of the synthesis process. Additionally, the purity of Tosylmethyl isocyanide achieved is 98.5%, underscoring the effectiveness of the purification steps integrated into the synthesis protocol.
References:
[1] CHRISTOPHER J DE FEO C D W. Escape from human immunodeficiency virus type 1 (HIV-1) entry inhibitors.[J]. ACS Applied Electronic Materials, 2012. DOI:10.3390/v4123859.You may like
Related articles And Qustion
Lastest Price from Tosylmethyl isocyanide manufacturers

US $1.00/KG2025-04-21
- CAS:
- 36635-61-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 36635-61-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month