Tosylmethyl isocyanide: applications in organic synthesis and safety
General Description
Tosylmethyl isocyanide is a colorless to yellowish liquid has a unique smell, which is a versatile reagent used in organic synthesis. It finds application in the synthesis of heterocyclic compounds, such as pyrroles, quinolines, and indoles, enabling the creation of structurally diverse molecules. Tosylmethyl isocyanide is also utilized in ketone synthesis, participating in reactions like the Pinner reaction and Strecker synthesis to produce α-acyloxyketones and α-amino ketones, respectively. Safety is crucial when working with tosylmethyl isocyanide, as it is hazardous if ingested or inhaled. Proper procedures, personal protective equipment, and ventilation should be employed to minimize risks.

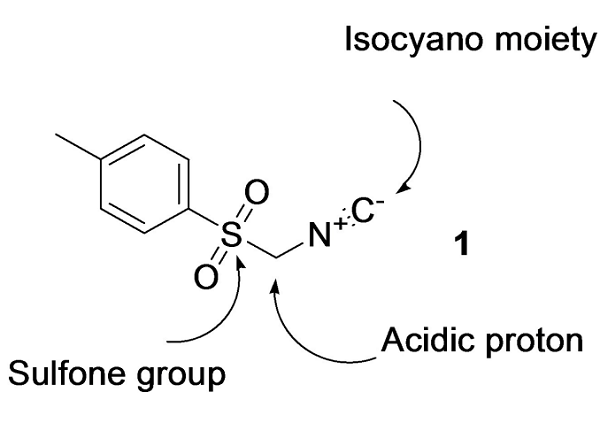
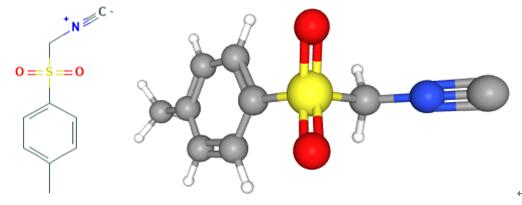
Figure 1. Tosylmethyl isocyanide
Applications in organic synthesis
Synthesis of heterocyclic compounds
Tosylmethyl isocyanide has proven to be a valuable reagent in the synthesis of various heterocyclic compounds. One major application of tosylmethyl isocyanide is in the synthesis of pyrroles. Pyrroles are five-membered rings containing one nitrogen atom and four carbon atoms. Tosylmethyl isocyanide undergoes a three-component reaction with an aldehyde and an amine, known as the Pictet-Spengler reaction, leading to the formation of pyrrole derivatives. This method provides a rapid and efficient route to access structurally diverse pyrrole-based compounds. Through a reaction called the Hantzsch dihydropyridine synthesis, tosylmethyl isocyanide reacts with an aldehyde or ketone, ammonia, and an α,β-unsaturated compound, yielding substituted pyridine derivatives. This allows for the synthesis of a range of functionalized pyridine molecules. Furthermore, tosylmethyl isocyanide can be employed in the synthesis of other heterocycles, such as quinolines and indoles, through various multi-step reactions. Overall, the versatile nature of tosylmethyl isocyanide makes it an essential tool in the synthesis of heterocyclic compounds. 1
Synthesis of ketone
Tosylmethyl isocyanide is widely used in the synthesis of ketones, which are important organic compounds containing a carbonyl group (C=O) bonded to two carbon atoms. Tosylmethyl isocyanide finds application in several key reactions for efficient ketone synthesis. One significant use of tosylmethyl isocyanide is in the Pinner reaction. By reacting with alcohols and acid chlorides or anhydrides in the presence of a Lewis acid catalyst, tosylmethyl isocyanide enables the formation of α-acyloxyketones. This reaction provides a straightforward method to introduce acyloxy groups into ketone structures, making it valuable in natural product synthesis and pharmaceutical development. Tosylmethyl isocyanide also plays a role in the Strecker synthesis, where it reacts with imines derived from aldehydes and amines. Subsequent hydrolysis leads to the formation of α-amino ketones. This synthesis route is widely employed for generating α-amino ketones, which are important intermediates for various bioactive molecules like amino acids and pharmaceuticals. Additionally, tosylmethyl isocyanide can participate in the synthesis of β-keto sulfoxides through reactions with sulfonium salts. The resulting sulfoxonium ylides undergo rearrangement to yield β-keto sulfoxides. In summary, tosylmethyl isocyanide plays a crucial role in diverse ketone synthesis methods, enabling the creation of versatile ketone frameworks with applications in various fields. 2
Safety
Tosylmethyl isocyanide is a hazardous chemical that poses risks if ingested or inhaled. It can lead to allergies, asthma symptoms, respiratory difficulties, and reproductive toxicity. To ensure safe handling, it is crucial to adhere to proper procedures and wear personal protective equipment. Tosylmethyl isocyanide should be used outdoors or in well-ventilated areas to prevent inhalation. It has irritant properties and may cause skin, eye, and respiratory irritation. Therefore, wearing goggles, gloves, and protective clothing is essential. Tosylmethyl isocyanide is also flammable and can ignite or explode when exposed to high temperatures or flames. Hence, it is vital to avoid ignition sources and maintain adequate ventilation and fire safety measures. In the event of a leak, isolate the area, clean it with absorbent materials, and avoid direct contact while wearing protective gear. Safety precautions and responsible handling are paramount when working with tosylmethyl isocyanide. 3
Reference
1. Coppola A, Sucunza D, Burgos C, Vaquero JJ.
Isoquinoline synthesis by heterocyclization of tosylmethyl isocyanide
derivatives: total synthesis of mansouramycin B. Org Lett, 2015,
17(1):78-81.
2. Zhang J, Gao Q, Wu X, Geng X, Wu YD, Wu A. Dual Roles of Methyl Ketones in Radziszewski-Type Reaction: Formal [2+1+1+1] Synthesis of 1,2,5-Trisubstituted Imidazoles. Org Lett, 2016, 18(7):1686-1689.
3. Nauth AM, Opatz T. Non-toxic cyanide sources and cyanating agents. Org Biomol Chem, 2018, 17(1):11-23.
Related articles And Qustion
See also
Lastest Price from Tosylmethyl isocyanide manufacturers

US $1.00/KG2025-04-21
- CAS:
- 36635-61-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $6.00/kg2025-04-21
- CAS:
- 36635-61-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month