Pyromellitic dianhydride: applications and toxicology
General Description
Pyromellitic dianhydride has various applications in polymer synthesis, wastewater treatment, solar cell fabrication, but it also has toxicity concerns. Pyromellitic dianhydride is used to create new water-soluble amino β-cyclodextrin-based polymers and boron nitride composites for pollutant removal. It is also utilized in the manufacture of dye-sensitized solar cells. However, occupational asthma cases have been reported among workers exposed to pyromellitic dianhydride. It's important to address its toxicity and ensure safety in occupational settings.

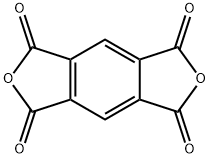
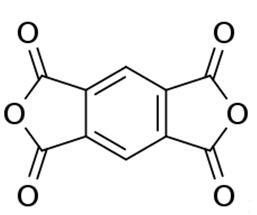
Figure 1. Pyromellitic dianhydride
Applications
Synthesis of β-cyclodextrin-based polymer
Pyromellitic dianhydride was utilized in the synthesis of a new water-soluble amino β-cyclodextrin-based polymer. Amino derivatives of β-cyclodextrins (β-CDs) were created by attaching amino groups to β-CDs using mono-6-azido-6-deoxy-β-cyclodextrin (β-CD-N3) and triphenylphosphine (Ph3P). An amino blocking reaction was performed, followed by purification of the resulting polymer through ultrafiltration. Furthermore, an attempt was made to form nanospheres by encapsulating the polymer with chitosan (CT) under acidic conditions, yielding nanospheres for the first time in this reaction. Scanning electron microscopy (SEM), 1H nuclear magnetic resonance (NMR), electrospray ionization mass spectrometry (ESI-MS), and differential scanning calorimetry (DSC) techniques were employed for product confirmation and structural characterization. These findings demonstrate the potential application of pyromellitic dianhydridein the synthesis of novel polymers and development of nanospheres through its reaction with amino β-cyclodextrin-based polymers and chitosan. 1
Pollutant removal from wastewater
Pyromellitic dianhydride was used to synthesize boron nitride-pyromellitic dianhydride composites. The composite BNPA2 showed high adsorption capacity for rhodamine B (RhB) and effective photo-removal of various pollutants under visible light. BNPA2 also exhibited adsorption capabilities for neutral red (NR), methyl orange (MO), tetracycline (TC), and atrazine (AT). It selectively removed cationic dyes (RhB and NR) from mixtures with anionic dye MO, indicating electrostatic interactions play a significant role. The photo-removal of pollutants by BNPA2 was mainly attributed to superoxide ions (O2-) and the material could be regenerated by visible light. Overall, pyromellitic dianhydride -based composites like BNPA2 have potential for efficient pollutant removal under visible light conditions. 2
Fabrication of solar cell
Pyromellitic dianhydride has been applied in the manufacture of dye-sensitized solar cells. By synthesizing a supramolecular hydrogel called PMDIG through acid-base treatment, pyromellitic dianhydride forms a fibrillar network structure. PMDIG gel exhibits enhanced fluorescence compared to its sodium salt. It can transform into a sol state with anilinium chloride solution and form a stable hydrogel with solid ammonium persulfate. PMDIG can also co-assemble with polyaniline (PANI), resulting in improved mechanical properties such as higher storage modulus, elasticity, stiffness, and fragility. Spectroscopic and X-ray scattering analyses confirm the interaction between PANI and PMDIG molecules in the PMDIG-PANI hydrogel. Additionally, the PMDIG-PANI xerogel exhibits significantly increased dc conductivity and shows rectification properties under white light illumination, indicating photocurrent rectification. This makes it suitable for enhancing the performance of dye-sensitized solar cells. The PMDIG-PANI hydrogel has been employed in the fabrication of such solar cells, achieving a power conversion efficiency of 0.1%. 3
Toxicology
Pyromellitic dianhydride has been implicated in causing occupational asthma, as seen in three workers from a plastic foil manufacturing plant. These workers developed asthma symptoms after exposure to pyromellitic dianhydride during specific inhalation challenges (SIC). The SIC tests revealed a delayed decrease in forced expiratory flow in 1 second (FEV1) in all cases, indicating sensitization to pyromellitic dianhydride. The exact mechanism of anhydride-related asthma is not fully understood, but it is believed that both irritative and sensitizing responses may play a role. To mitigate the risk, the company improved ventilation, enforced the use of respiratory protection equipment, and eventually phased out pyromellitic dianhydride. Occupational risk assessment and SIC testing can help identify and confirm asthma-inducing substances in the workplace, leading to improved working conditions. 4
Reference
1. Kozie? K, ?agiewka J, Girek B, Folentarska A, Girek T, Ciesielski W. Synthesis of New Amino-β-Cyclodextrin Polymer, Cross-Linked with Pyromellitic Dianhydride and Their Use for the Synthesis of Polymeric Cyclodextrin Based Nanoparticles. Polymers (Basel), 2021, 13(8):1332.
2. Guo Y, Wang R, Yan C, Wang P, Rao L, Wang C. Developing boron nitride-pyromellitic dianhydride composite for removal of aromatic pollutants from wastewater via adsorption and photodegradation. Chemosphere, 2019, 229:112-124.
3. Bairi P, Chakraborty P, Shit A, Mondal S, Roy B, Nandi AK. A co-assembled gel of a pyromellitic dianhydride derivative and polyaniline with optoelectronic and photovoltaic properties. Langmuir, 2014, 30(25):7547-7555.
4. Madsen MT, Skadhauge LR, Nielsen AD, Baelum J, Sherson DL. Pyromellitic dianhydride (PMDA) may cause occupational asthma. Occup Environ Med, 2019, 76(3):175-177.
Related articles And Qustion
Lastest Price from Pyromellitic Dianhydride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 89-32-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 89-32-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500mt/year