Tosyl chloride: Application, Preparation
Indication
Tosyl chloride, also known as toluenesulfonyl chloride, is an organic compound with the chemical formula CH3C6H4SO2Cl. It is a colorless to pale yellow liquid that is used in organic chemistry as a reagent for converting alcohols and amines into corresponding sulfonate esters or amides, respectively. Tosyl chloride reacts with primary amines to form N-tosylamines, which are used as intermediates in the synthesis of pharmaceuticals, agrochemicals, and other fine chemicals. Tosyl chloride is also used as a protecting group for alcohols and amines in organic synthesis[1]. It is an important building block in organic chemistry and is widely used in the pharmaceutical industry.

Figure 1 Appearance of Tosyl chloride
Application
Tosyl chloride is a versatile reagent that is widely used in organic synthesis. Tosyl chloride can be used to protect alcohols by converting them to the corresponding tosylates. These tosylates can be easily deprotected later using mild conditions. It reacts with amines to form sulfonamides, which are important intermediates in the synthesis of pharmaceuticals and agrochemicals[2]. Tosylhydrazones are important intermediates in the synthesis of various compounds such as hydrazones, β-lactams, and azines. Aryl and vinyl sulfones can be prepared by reacting aryl or vinyl halides with sodium sulfite in the presence of Tosyl chloride. Tosyl chloride can be used to promote cyclization reactions, especially when a good leaving group is needed. It can be used as a catalyst for the formation of carbon-carbon bonds, for example, in the Friedel-Crafts reaction. It can be used as an oxidizing agent in certain reactions, such as the oxidation of aldehydes to carboxylic acids[3].
Tosyl chloride can be used to prepare esters and amides from carboxylic acids and amines or alcohols, respectively. It can also be used to protect amines by forming the corresponding tosylamines, which can be easily deprotected later. Tosyl chloride is frequently used in the synthesis of heterocyclic compounds, such as pyrazoles, pyrimidines, and pyridines. Tosyl chloride is commonly used in the synthesis of β-lactams, which are important building blocks for the synthesis of antibiotics and other bioactive compounds. Thioethers can be prepared by reacting thiols with alkyl or aryl halides in the presence of Tosyl chloride. It can be used in the oxidative cleavage of alkenes to form carbonyl compounds, via a Baeyer-Villiger oxidation mechanism. Tosyl chloride can be used in glycosylation reactions to protect the hydroxyl group of carbohydrates, facilitating the formation of glycosidic bonds[4]. N-acylureas can be prepared by reacting isocyanates with carboxylic acids in the presence of Tosyl chloride. Carboxylic acids can be protected as their corresponding tosylates, allowing them to be used as intermediates in various synthetic pathways. It can be used in the synthesis of β-enaminones, which are important intermediates in the synthesis of various heterocyclic compounds. Tosyl chloride can be converted to tosylate salts, known as triflates, which are valuable leaving groups in organic synthesis. Tosyl chloride can be used to protect the hydroxyl group in phosphonic acids by forming tosylates, facilitating their use in synthetic pathways. It can be used to prepare aryl azides, which are versatile intermediates for the synthesis of a wide range of compounds. It can be used to prepare alkyl fluorides from the corresponding alcohols or alkyl halides in the presence of a fluoride source such as KF. It can be used in the synthesis of imidazoles, which are important heterocyclic compounds with a wide range of applications. It can be used in dehydration reactions to remove water and promote the formation of double bonds. Tosyl chloride can be used in the synthesis of nitrones, which are reactive intermediates that have been used in various synthetic transformations[5]. Tosyl chloride is a valuable reagent in organic synthesis due to its versatility and ease of use.
Preparation
The synthesis of Tosyl chloride is shown in Figure 2, using toluene as the raw material, chlorosulfonic acid, phosphorus oxychloride as sulfonating agents, and inorganic ammonium salts as sulfonating aids. The raw material toluene undergoes a chlorosulfonation reaction under the action of sulfonating agents chlorosulfonic acid, phosphorus oxychloride, and sulfonation auxiliaries. The reaction liquid is hydrolyzed at low temperature, and then subjected to solvent extraction, water washing, and water separation to remove the water layer. The oil phase is precipitated, filtered, and dried at less than 5 ℃, and the solid is p-toluenesulfonyl chloride. The filtrate is subjected to vacuum distillation to remove the solvent, and a sulfonated oil based on p-toluenesulfonyl chloride is obtained. The total yield can reach 98.8%[6].
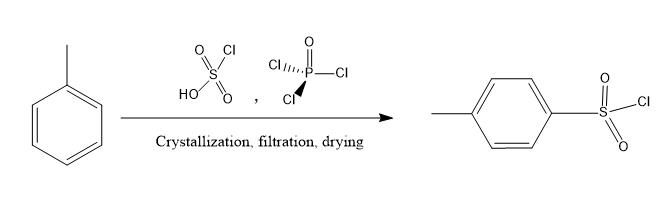
Figure 2 preparation of Tosyl chloride
References
[1] Nayad A, Hasnaoui A, Fkhar L, et al. New two-dimensional functionalised silicon nanosheets prepared by direct exfoliation of calcium disilicide with tosyl chloride[J]. Advances in Materials and Processing Technologies,2022,8(3).
[2] Dykun Oleksii M, Anishchenko Viktor M, Redko Andrii M, et al. Spectroscopic study of stepwise gossypol sulfonylation with tosyl chloride in the presence of 4-methoxypyridine N-oxide and triethylamine[J]. Journal of Molecular Structure,2021,1246.
[3] Farrell Robert P.,Tedrow Jason S.,Silva Elipe Maria. Development of a highly regio-selective, one-pot amination of 3, 5-disubstituted pyridine-N-oxides using saccharin as an ammonia surrogate via tosyl chloride activation[J]. Abstracts of Papers of the American Chemical Society. 2012,244.
[4] Chemistry - Organic Chemistry; Findings from Deemed University Provides New Data on Organic Chemistry (A Simple Synthesis of Ketone From Carboxylic Acid Using Tosyl Chloride As an Activator)[J]. Chemicals & Chemistry,2019.
[5] Novokshonov Vladimir V.,Xuan Nguyen Thi Thu,Shaglaeva Nina S.,Podgorbunskaya Tatiana A.,Bayandin Victor V.. Interaction of β-cyclodextrin with tosyl chloride in an aqueous alkaline medium[J]. proceedings of Universities Applied chemistry and Biotechnology,2019,9(3).
[6] A new process for the synthesis of toluenesulfonyl chloride Zhejiang University, Zhejiang Province, January 1, 2009.
You may like
Related articles And Qustion
Lastest Price from Tosyl chloride manufacturers

US $1.00/PCS2025-04-21
- CAS:
- 98-59-9
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 98-59-9
- Min. Order:
- 1Kg/Bag
- Purity:
- 99.5%
- Supply Ability:
- 1000mt/year