What is TsCl used for?Is it Toxic?
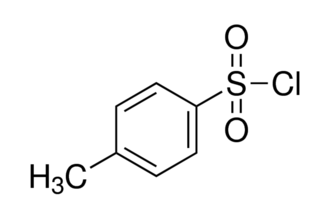
4-Toluenesulfonyl chloride is a white, malodorous solid, with the formula CH3C6H4SO2Cl. It is widely used in organic synthesis as a reagent. Abbreviated TsCl, it is a derivative of toluene and contains a sulfonyl chloride (−SO2Cl) functional group.

Uses
Being a widely available reagent, TsCl is used in dehydrations to make nitriles, isocyanides, and diimides. In an unusual reaction focusing on the sulfur center, zinc reduces TsCl to the sulfinate, CH3C6H4SO2Na.
Synthesis
TsCl is inexpensively available for laboratory use. It is a by-product from the production of ortho-toluenesulfonyl chloride (a precursor for the synthesis of the common food additive and catalyst saccharin), via the chlorosulfonation of toluene:
CH3C6H5 + SO2Cl2 → CH3C6H4SO2Cl + HCl
Toxicity
TsCl is considered to be a toxic and corrosive substance.
Like many chemical reagents, TsCl should be handled with care and appropriate safety precautions. It is important to wear protective equipment, such as gloves and goggles, and work in a well-ventilated area when using TsCl.
Effects of short-term exposure
TsCl is severely irritating to the eyes.TsCl is irritating to the skin and respiratory tract.
You may like
Related articles And Qustion
See also
Lastest Price from Tosyl chloride manufacturers

US $1.00/PCS2025-04-21
- CAS:
- 98-59-9
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 98-59-9
- Min. Order:
- 1Kg/Bag
- Purity:
- 99.5%
- Supply Ability:
- 1000mt/year