What is Tosyl chloride?
General Description
Tosyl chloride is a white to gray powdered solid with a distinctive odor. Insoluble in water and denser than water. Contact may irritate skin, eyes and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
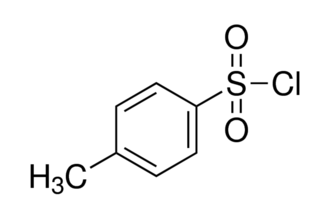
Tosyl chloride is an important intermediate dyes in organic synthesis and raw materials for pesticides, there are three kinds of isomers, namely o-toluenesulfonyl chloride, m-toluenesulfonyl chloride and p-toluenesulfonyl chloride. Relative molecular mass is 190.65. All three stimulate skin and mucous membranes, commonly used is adjacent toluenesulfonyl chloride and p-toluenesulfonyl chloride. O-toluenesulfonyl chloride, also known as 2-methyl-benzenesulfonyl chloride, 2-toluene-sulfonyl chloride, is colorless oily liquid. It is insoluble in water, soluble in ether, benzene and ethanol. M-toluenesulfonyl chloride, also known as 3-methyl-benzenesulfonyl chloride, 3-toluenesulfonyl chloride, is colorless oily liquid. It is insoluble in water, soluble in alcohol, ether and benzene.
P-toluenesulfonyl chloride is also known as 4-methyl-benzenesulfonyl chloride, 4-toluenesulfonyl chloride. Precipitation from ether or petroleum ether is triclinic white flaky crystal. It is insoluble in water, soluble in alcohol, benzene and ether.
Toluenesulfonyl chloride is called "TsCl", with strong nucleophilicity and substitution reaction with nucleophilic reagent. For example, reaction with the alcohol into the ester:
ROH + TsCl-Py → ROTs + Py + HCl-, reaction with amine and hydrazine respectively to generate the sulfonamide and sulfonyl hydrazide:
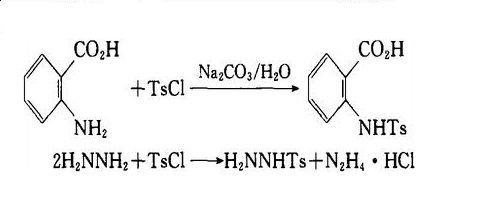
Chemical Properties
White flaky crystal. melting point is 71 ℃. Boiling point is 151.6 ℃ (1.67kPa), 145-146 ℃ (2.0kPa). Soluble in alcohol, ether and benzene, insoluble in water.
Reactions
In characteristic manner, TsCl converts alcohols (abbreviated ROH) into the corresponding toluenesulfonate esters, or tosyl derivatives ("tosylates"):
CH3C6H4SO2Cl + ROH → CH3C6H4SO2OR + HCl
Tosylates can be cleaved with lithium aluminium hydride:
4 CH3C6H4SO2OR + LiAlH4 → LiAl(O3SC6H4CH3)4 + 4 RH
Thus, tosylation followed by reduction allows for removal of a hydroxyl group.
Likewise, TsCl is used to prepare sulfonamides from amines:
CH3C6H4SO2Cl + R2NH → CH3C6H4SO2NR2 + HCl
The resulting sulfonamides are non-basic and, when derived from primary amines, are even acidic.
The preparation of tosyl esters and amides are conducted in the presence of a base, which absorbs hydrogen chloride. The selection of the base is often crucial to the efficiency of tosylation. Typical bases include pyridine and triethylamine. Unusual bases are also used; for example, catalytic amounts of trimethylammonium chloride in the presence of triethylamine is highly effective by virtue of the trimethylamine.
Uses
Tosyl chloride is mainly used in the preparation of chemical derivatives in the pharmaceutical, plastics and organic chemical industries, as an intermediate for disperse dyes, azoic dyes, acid dyes. Also used in the production of drugs homosulfanilamide. It is used for the analysis reagents, but also for organic synthesis, dye preparation and molecule rearrangement in the hormone synthesis. Used for organic synthesis, sulfa drugs and as pesticide intermediates.
p-Toluenesulfonyl chloride (tosyl chloride or p-TsCl) is an organic sulfonyl chloride mainly used to convert hydroxyl and amine groups into good leaving groups by forming sulfonates.p-Toluenesulfonyl chloride can be used as:
• An additive to enhance the yield of symmetrical biaryls via palladium chloride catalyzed homo-coupling of aryl boronic acids in the absence of ligands.
• A chlorine source for the α-chlorination of ketones in the presence of LDA.
• A reactant in the tosylation of alcohols and phenols in the presence of heteropoly acids.
• An activator for the reaction between 2-alkynylbenzaldoxime and phenols to form 1-aroxyisoquinolines in the presence of silver triflate.
• A catalyst for the solvent-free preparation of symmetrical bis(benzhydryl)ethers from benzhydrols.
Production method
Toluene chlorosulfonation production into o-toluenesulfonyl chloride, at the same time p-toluenesulfonyl chloride is also generated. The filter cake was separated from the o-toluenesulfonyl chloride, refined to obtain p-toluenesulfonyl chloride.
You may like
Related articles And Qustion
See also
Lastest Price from Tosyl chloride manufacturers

US $1.00/PCS2025-04-21
- CAS:
- 98-59-9
- Min. Order:
- 1PCS
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 98-59-9
- Min. Order:
- 1Kg/Bag
- Purity:
- 99.5%
- Supply Ability:
- 1000mt/year




