Orthoboric Acid: Applications, safety and Synthesis
Introduction
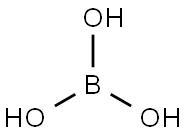
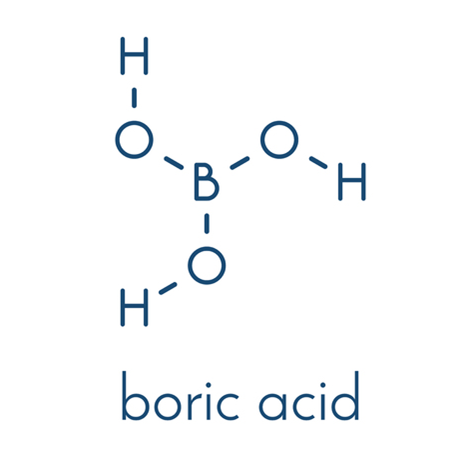
Orthoboric acid[1], also known as hydrogen borate, is a weak acid of boron with the chemical formula H3BO3. It is found in nature as a mineral called sassolite and is available commercially as a white crystalline powder or colorless crystals. Orthoboric acid has various properties such as antiseptic, fungistatic, insecticide, and flame retardant, which make it useful in several applications. It was first isolated in 1702 by Wilhelm Homberg, who extracted it from a mineral called borax. Since then, it has been used for various purposes, including as an antiseptic, insecticide, fungicide, and flame retardant. Orthoboric acid is also known for its medicinal properties, such as the ability to treat eye infections and certain skin conditions. Orthoboric acid is a naturally occurring compound that has been used for centuries in many different ways.

Figure 1 Physical picture of Orthoboric acid
Application
Orthoboric acid finds its use in diverse applications:
1. Insecticides: Its insecticidal properties make it an important component of cockroach and ant baits, controlling termites, fleas, and other insects. Orthoboric acid is often used as an insecticide because of its toxicity to insects. It works by disrupting their nervous systems and causing dehydration, leading to death. Orthoboric acid is often incorporated into baits or sprayed directly onto surfaces to control pests such as cockroaches, ants, termites, fleas, and silverfish.
2. Flame retardants: Orthoboric acid is used to flame-proof products like cellulose insulation, textiles, plastics, and wood. Orthoboric acid is an effective flame retardant, which means it can prevent fires or slow down their spread. It does this by releasing water when exposed to heat, which cools the surrounding area and prevents ignition. Orthoboric acid is commonly used in cellulose insulation, textiles, plastics, and wood products.
3. Antiseptics: It is used as an antiseptic for minor cuts, burns, and other skin wounds. Orthoboric acid's antiseptic properties make it useful in treating minor cuts, burns, and other skin wounds. It is often used in combination with other ingredients, such as alcohol or hydrogen peroxide, to create a solution that can be applied topically.
4. Fungicides: Orthoboric acid has fungistatic properties that make it useful in treating fungal infections such as athlete's foot and yeast infections. Orthoboric acid has fungistatic properties, meaning it can inhibit the growth of fungi. It is commonly used in the treatment of fungal infections such as athlete's foot and yeast infections.
5. Nuclear power plants: Orthoboric acid is used as a coolant in nuclear power plants. Orthoboric acid is used as a coolant in nuclear power plants due to its ability to absorb neutrons. This helps regulate the rate of nuclear reactions and prevent overheating.
In addition to its practical uses, Orthoboric acid has also found applications in research and industry. For example, it is used as a flux in metallurgy and as a chemical intermediate in the production of boron compounds. Orthoboric acid has also been studied extensively for its potential as a therapeutic agent, particularly in the treatment of cancer and other diseases[2].
Safety
While Orthoboric acid is generally regarded as safe when used properly, it can pose health risks if ingested or inhaled in large amounts. Ingestion of Orthoboric acid can cause nausea, vomiting, abdominal pain, and diarrhea. In extreme cases, it can lead to kidney damage, liver damage, and even death. Inhalation of Orthoboric acid can cause respiratory irritation, coughing, and difficulty breathing. Therefore, proper handling and storage of Orthoboric acid is essential. It should be kept out of reach of children and pets, and protective clothing should be worn when handling it. Additionally, Orthoboric acid should not be used in food preparation, and products containing Orthoboric acid should be labeled appropriately. In conclusion, Orthoboric acid is a versatile compound with many practical applications, including as an insecticide, flame retardant, antiseptic, and fungicide. Its diverse range of properties has made it useful in research and industry, and it continues to be studied for its potential therapeutic uses. However, proper handling and storage are essential to prevent health risks associated with ingestion or inhalation[3].
Synthesis
Orthoboric acid can be synthesized using various methods[4], including reaction of borax with an acid or boron oxide with water vapor. The most common method involves reacting borax with hydrochloric acid, sulfuric acid, or another acid:
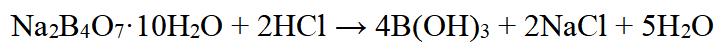
Figure 2 Chemical reaction of preparation for Orthoboric acid
Another method involves heating boron trioxide with water vapor:
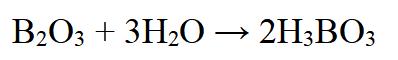
Figure 3 Chemical reaction of preparation for Orthoboric acid
References
[1] R. W. Olsen and N. M. Ford, "Orthoboric Acid," Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., 2010.
[2] E. J. Behrman and J. G. Hawley, "Orthoboric Acid," Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2005.
[3] S. A. Lawrence, "Orthoboric Acid," in Amino Acids, Peptides and Proteins in Organic Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2011, pp. 193-199.
[4] K. E. R. Marriott and A. T. Beyersmann, eds., "Boron," in Encyclopedia of Inorganic and Bioinorganic Chemistry, John Wiley & Sons, Ltd., 2016, pp. 1-37.
Related articles And Qustion
See also
Lastest Price from Boric acid manufacturers

US $1.00/kg2025-04-21
- CAS:
- 10043-35-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons

US $0.00/kg2024-06-11
- CAS:
- 10043-35-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1 tons