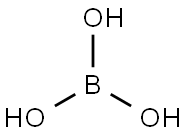
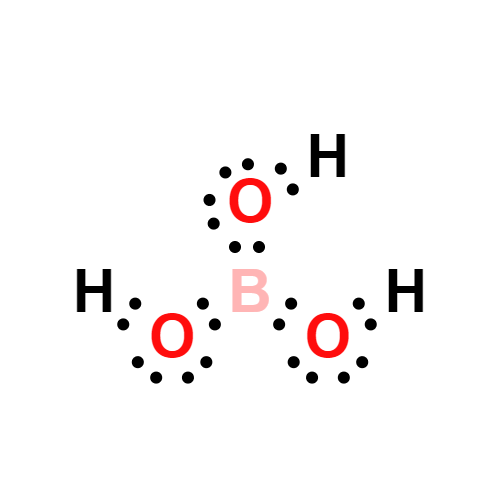
Description
Boric acid (orthoboric acid) is a weakly acidic hydrate of boric oxide with mild antiseptic, antifungal, and antiviral properties.
Boric acid can be used to treat yeast infections and acne, for eyewash by treating any bacterial infection and soothing inflamed eyes, and as a cleanser, deodorizer, stain remover, disinfectant and mold killer. Boric acid can be used as a pesticide to control a variety of pests, as a fungicide for citrus, and as an herbicide along rights-of-way. Boric acid can be used for the manufacture of textile fiberglass, household glass products and the glass used in LCD displays, to reinforce plastics in various products (boats, computer circuit boards and pipes), as a flame retardant, and as a pH buffer agent in plating.
References
[1] http://npic.orst.edu
[2] https://www.polyu.edu.hk
[3] Zenat A. Nagieb, Mona A. Nassar, Magda G. El-Meligy (2011) Effect of Addition of Boric Acid and Borax on Fire-Retardant and Mechanical Properties of Urea Formaldehyde Saw Dust Composites, International Journal of Carbonhydrate Chemistry, 2011, 146763
[4] http://www.boricacid.net.au/uses-of-boric-acid
Description
Boric acid is a white, amorphous powder orcolorless, crystalline solid. Molecular weight=61.84;Boiling point=300℃ (decomposes: loses 1/2 its H2O);Freezing/Melting point=170.9℃ (decomposes above100℃). Saturated solutions: at 0℃, 2.6% acid; at 100℃,28% acid. Hazard Identification (based on NFPA-704 MRating System): Health 2, Flammability 0, Reactivity 1.Boric acid is soluble in water; solubility=4.7 g/100 mL at20℃.
Chemical Properties
White powder or granules and odorless. It is incompatible with potassium, acetic anhydride, alkalis, carbonates, and hydroxides. Boric acid has uses in the production of textile fiberglass, flat panel displays, and eye drops. Boric acid is recognized for its application as a pH buffer and as a moderate antiseptic agent and emulsifier.
Chemical Properties
Boric acid occurs as a hygroscopic, white crystalline powder,
colorless shiny plates, or white crystals.
Chemical Properties
Boric acid is a white, amorphous powder or
colorless, crystalline solid.
Physical properties
Colorless, transparent triclinic crystal or white granule or powder; density 1.435 g/cm
3; melts at 171°C under normal heating; however, slow heating causes loss of water; sparingly soluble in cold water (4.7% at 20°C); pH of 0.1M solution 5.1; readily dissolves in hot water (19.1% at 80°C and 27.5% at 100°C); also soluble in lower alcohols and moderately soluble in pyridine.
Uses
It is a precursor material for other boron compounds. A dilute water solution of boric acid is usually employed as a mild antiseptic and eyewash. Boric acid is too employed in leather manufacture, electroplating, and cosmetics.
Uses
For weatherproofing wood and fireproofing fabrics; as a preservative; manufacture of cements, crockery, porcelain, enamels, glass, borates, leather, carpets, hats, soaps, artificial gems; in nickeling baths; cosmetics; printing and dyeing, painting; photography; for impregnating wicks; electric condensers; hardening steel. Also used as insecticide for cockroaches and black carpet beetles.
Uses
Boric acid can be used to study molecular biology, DNA and RNA purification, biological buffers and molecular biology reagents. Boric acid has been used to test the toxic effects of boron on growth and antioxidant system parameters of maize (
Zea mays L.) roots. Boric acid has also been used to study the effect of time period after boric acid injection on (10)B absorption in different regions of adult male rat′s brain.
Definition
ChEBI: Boric acid is a member of boric acids. It has a role as an astringent. It is a conjugate acid of a dihydrogenborate.
Production Methods
Boric acid occurs naturally as the mineral sassolite. However, the
majority of boric acid is produced by reacting inorganic borates
with sulfuric acid in an aqueous medium. Sodium borate and
partially refined calcium borate (colemanite) are the principal raw
materials. When boric acid is made from colemanite, the fineground
ore is vigorously stirred with mother liquor and sulfuric acid
at about 908℃. The by-product calcium sulfate is removed by
filtration, and the boric acid is crystallized by cooling the filtrate.
Preparation
Boric acid is produced from borax, colemanite, or other inorganic borates by reaction with sulfuric acid or hydrochloric acid, and cooling the solution to proper temperature:
Na
2B
4O
7 ? 10Η
2Ο + H
2SO
4 → 4H
3BO
3 + Na
2SO
4 + 5H
2O
It also may be prepared by extraction of weak borax brine with a kerosene solution of an aromatic diol, such as 2-ethyl-1,3-hexanediol or 3-chloro- 2-hydroxy-5-(1,1,3,3-tetramethylbutyl)benzyl alcohol. The diol-borate chelate formed separates into a kerosene phase. Treatment with sulfuric acid yields boric acid which partitions into aqueous phase and is purified by recrystallization.
brand name
Alpagelle;Anojel;Anugard;Anojel;Anugard;Anusol hc;Anusol hc;Berlicetin;Betadrin;Berlicetin;Betadrin;Bluboro;Boroformal;Bluboro;Boroformal;Borogal;Borogal;Borsyre viskos;Cacimag;Borsyre viskos;Cacimag;Caclcifor;Caclcifor;Calcamyl-24;Calcibenzamin;Calcamyl-24;Calcibenzamin;Camilca;Camilca;Chibro;Coneolent;Chibro;Coneolent;Cutaden;Cutaden;Dissol;Ear-dry;Dissol;Ear-dry;Egosol-bs;Egosol-bs;Evercil;Fermakzem;Evercil;Fermakzem;Flex-care;Flex-care;Glaucadrine;Glucocalcium;Glaucadrine;Glucocalcium;Kalopsisi;Kerapos;Kalopsisi;Kerapos;Kodomo smarin;Kodomo smarin;Komex;Lindemil;Komex;Lindemil;Macaldex;Macaldex;Mentol sedans sulfamidad;Neo-smarin dia;Mentol sedans sulfamidad;Neo-smarin dia;Neo-vagipurin;Neo-vagipurin;Normol;O-biol;Normol;O-biol;Oestro-gynedron;Oestro-gynedron;Ophtalmin;Otocaina;Ophtalmin;Otocaina;Pedoz;Pedoz;Phoscanol;Phoscanol;Poly-gynedron;Preferal;Poly-gynedron;Preferal;Proculin;Proculin;Rhinophenazol;Saddle mate;Rhinophenazol;Saddle mate;Swim-ear;Swim-ear;Swim-eye;Swim-eye;Timazincum;Timazincum;Tipolin;Tricho-gynedron;Tipolin;Tricho-gynedron;Unisol;Unisol;Vetacalin-m;Alpagelle;Vetacalin-m.
World Health Organization (WHO)
Boric acid and some borates were formerly extensively used as
disinfectants and antiinflammatory agents. By the late 1960s an association between
the death of many infants and application of high concentrations of boric acid
contained in topical preparations used in the treatment of napkin rash had been
established. This led to the restriction of the use of boric acid in pharmaceutical
preparations by many regulatory authorities. In some countries it is now permitted
only as an ingredient in ophthalmological preparations.
General Description
Boric acid is a weak monobasic acid, it accepts OH
- ions, hence is a Lewis acid. In boric acid, B is sp
2 hybridized, forming a planar triangle structure. The principal oxide of boron, B
2O
3, is obtained as a vitreous solid by dehydration of boric acid at red heat.
Hazard
Toxic via ingestion. Use only weak solu-
tions. Irritant to skin in dry form.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Boric acid is used as an antimicrobial preservative in eye drops,
cosmetic products, ointments, and topical creams. It is also used as
an antimicrobial preservative in foods.
Boric acid and borate have good buffering capacity and are used
to control pH; they have been used for this purpose in external
preparations such as eye drops.
Boric acid has also been used therapeutically in the form of
suppositories to treat yeast infections. In dilute concentrations it
is used as a mild antiseptic, with weak bacteriostatic and fungistatic
properties, although it has generally been superseded by more
effective and less toxic disinfectants.
Biochem/physiol Actions
Boric acid has antibacterial and fungicidal properties. It is used in the periodontal therapy as an irrigation solution as it elicits bactericidal effects in microbial biofilms in root canal. Boric acid may favor osteoblastic activity and inhibit bone loss. It inhibits Candida albicans fungal infection and has potential to treat vaginal infection.
Safety
Boric acid is a weak bacteriostatic and antimicrobial agent, and has
been used in topical preparations such as eye lotions, mouthwashes
and gargles. It has also been used in US- and Japanese-approved
intravenous products. Solutions of boric acid were formerly used to
wash out body cavities, and as applications to wounds and ulcers,
although the use of boric acid for these purposes is now regarded as
inadvisable owing to the possibility of absorption. Boric acid is
not used internally owing to its toxicity. It is poisonous by ingestion
and moderately toxic by skin contact. Experimentally it has proved
to be toxic by inhalation and subcutaneous routes, and moderately
toxic by intraperitoneal and intravenous routes.
Boric acid is absorbed from the gastrointestinal tract and from
damaged skin, wounds, and mucous membranes, although it does
not readily permeate intact skin. The main symptoms of boric acid
poisoning are abdominal pain, diarrhea, erythematous rash
involving both skin and mucous membrane, and vomiting. These
symptoms may be followed by desquamation, and stimulation or
depression of the central nervous system. Convulsions, hyperpyrexia,
and renal tubular damage have been known to occur.
Death has occurred from ingestion of less than 5 g in young
children, and of 5–20 g in adults. Fatalities have occurred most
frequently in young children after the accidental ingestion of
solutions of boric acid, or after the application of boric acid powder
to abraded skin.
The permissible exposure limit (PEL) of boric acid is 15 mg/m
3
total dust, and 5 mg/m
3 respirable fraction for nuisance dusts.
Ld
Lo (man, oral): 429 mg/kg
Ld
Lo (woman, oral): 200 mg/kg
Ld
Lo (infant, oral): 934 mg/kg
Ld
Lo (man, skin): 2.43 g/kg
Ld
Lo (infant, skin): 1.20 g/kg
LD
50 (mouse, oral): 3.45 g/kg
LD
50 (mouse, IV): 1.24 g/kg
LD
50 (mouse, SC): 1.74 g/kg
LD
50 (rat, oral): 2.660 g/kg
LD
50 (rat, IV): 1.33 g/kg
LD
50 (rat, SC): 1.4 g/kg
Potential Exposure
Boric acid is a fireproofing agent for
wood; a preservative, and an antiseptic. It is used in the
manufacture of glass, pottery, enamels, glazes, cosmetics,
cements, porcelain, borates, leather, carpets, hats, soaps;
artificial gems; in tanning leather; printing, dyeing, painting,
and photography.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
storage
Boric acid is hygroscopic and should therefore be stored in an airtight,
sealed container. The container must be labeled ‘Not for
Internal Use’.
Shipping
UN 3077 Environmentally hazardous substances,
solid, n.o.s., Hazard class: 9; Labels: 9—Miscellaneous
hazardous material, Technical Name Required.
Purification Methods
Crystallise the acid three times from H2O (3mL/g) between 100o and 0o, after filtering through sintered glass.Dry it to constant weight over metaboric acid in a desiccator. It is steam volatile. After two recrystallisations of ACS grade. it had Ag at 0.2 ppm. Its solubility (%) in H2O is 2.66 at 0o, 4.0 at 12o and 24 at 80o. At 100o it loses H2O to form metaboric acid (HBO2). When it is heated to redness or slowly to 200o, or over P2O5 in vacuo, it dehydrates to boric anhydride (B2O3) [1303-82-6] to give a white hard glass or crystals with m ~294o.The glass softens on heating and liquefies at red heat. It is an astringent, a fungicide and an antibacterial.
[McCulloch J Am Chem Soc 59 2650 1937, Kelly J Am Chem Soc 63 1137 1941, Taylor & Cole J Chem Soc 70 1926, Conti J Soc Chem Ind 44 343T 1925.]
Incompatibilities
Boric acid decomposes in heat above
100 C, forming boric anhydride and water. Boric acid is
hygroscopic; it will absorb moisture from the air. Boric
acid aqueous solution is a weak acid; incompatible with
strong reducing agents including alkali metals and metal
hydrides (may generate explosive hydrogen gas); acetic
anhydride, alkali carbonates, and hydroxides. Violent
reaction with powdered potassium metal, especially if
impacted. Attacks iron in the presence of moisture.
Incompatibilities
Boric acid is incompatible with water, strong bases and alkali
metals. It reacts violently with potassium and acid anhydrides. It
also forms a complex with glycerin, which is a stronger acid than
boric acid.
Toxics Screening Level
The initial threshold screening level (ITSL) for acute exposure to borates is 80 μg/m3 as boron (1-hour averaging time) based on the Michigan Department of Environmental Quality (MDEQ), Air Quality Division (AQD) Rule 336.1233 (1) and (2). With molecular weight adjustment, the ITSL for boric acid is 460 μg/m3 (1-hour averaging time).
Waste Disposal
Boric acids may be recovered
from organic process wastes as an alternative to disposal.
Regulatory Status
Accepted for use as a food additive in Europe. Included in the FDA
Inactive Ingredients Database (IV injections; ophthalmic preparations;
(auricular) otic solutions; topical preparations). Reported in
the EPA TSCA Inventory. In the UK, the use of boric acid in
cosmetics and toiletries is restricted. Included in the Canadian List
of Acceptable Non-medicinal Ingredients.