The uses of N, N-dimethylformamide dimethyl acetal in organic
Introduction
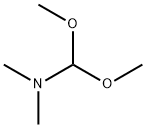
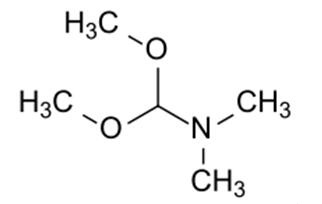
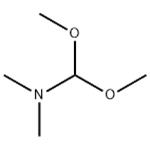
N, N-Dimethylformamide dimethyl acetal, also known as DMF-DMA or 1,1-dimethoxytrimethylamine, belongs to the class of organic compounds known as carboxylic acid amide acetals. These are carboxylic acid derivatives, two hydroxyl groups attached to a carbon atom, linked to a nitrogen atom(substituted or not). N-dimethylformamide dimethyl acetal is an acetal obtained by formal condensation of N, N-dimethylformamide with methanol. It is a derivatization agent used in gas-chromatography applications. It has a role as a chromatographic reagent. It is an acetal and a tertiary amino compound. It is functionally related to a N, N-dimethylformamide.
Related reactions
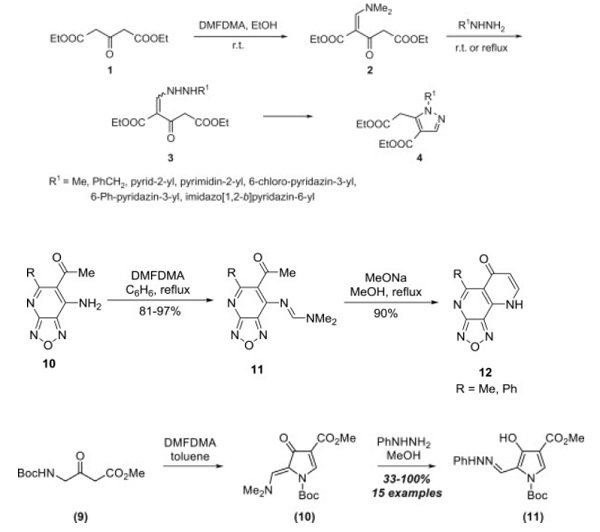
A. Diethyl acetone-1,3-dicarboxylate (1) reacts with N, N-dimethylformamide dimethyl acetal (DMFDMA) in ethanol at room temperature to give diethyl 1-dimethylamino-3-oxobut-1-ene-2,4-dicarboxylate (2), which was used without isolation and purification. To this mixture 1 equivalent of a monosubstituted hydrazine was added and then stirred at room temperature or heated under reflux for several hours to form intermediates 3, which were, without isolation, cyclized into 1-substituted 4-ethoxycarbonyl-5-(ethoxycarbonylmethyl)pyrazoles 4 in 24–71% yield (08ACSi1019).
B. Reaction of 6-acetyl-7-aminofurazano[3,4-b]pyridines (10) with N, N-dimethylformamide dimethyl acetal (DMFDMA) afforded N, N-dimethylformamidines (11) that were cyclized to the tricyclic furazan-fused [1,6]naphthyridine system (12) by treatment with sodium methoxide[1].
C. A series of 4-hydroxypyrroles was prepared by Grošelj and colleagues. β-Ketoester 9 was heated in toluene with N, N-dimethylformamide dimethyl acetal to furnish enaminone 10 after cyclization. Subsequent treatment of 10 with phenylhydrazine delivered 4-hydroxypyrrole 11 in moderate to excellent yields. Pyrroles such as 11 exist in equilibrium with their keto form and were successfully alkylated with benzyl bromide and methyl iodide. The intermediate enaminones could be isolated and characterized and proved helpful in synthesizing other functionalized heterocycles, including dihydropyridines, pyrazines, and pyrimidines.
D. Amide acetals can be accessed by transactivation of other amide acetals with alcohols. Typically, the starting material is N, N-dimethylformamide dimethyl acetal (1). The uncatalyzed reaction is driven to completion by removing generated methanol by distillation. The use of sterically hindered alcohols can, however, lower the reaction yields significantly. Cyclic amide acetals (2) are available from diols. Transamidation can be achieved with high-boiling amines such as morpholine and piperidine. Simultaneous transacetalation and transamidation have also been reported[2].
References
[1] Héctor Barbero“Bicyclic 5-6 Systems: Four Heteroatoms 1:3.”Comprehensive Heterocyclic Chemistry IV (2022): 395-418.
[2] RICE, M. J. “ChemInform Abstract: Functions Containing a Chalcogen and Any Other Heteroatoms Other Than a Halogen.”Comprehensive Organic Functional Group Transformations (1995): 103-136.
You may like
Related articles And Qustion
See also
Lastest Price from N,N-Dimethylformamide dimethyl acetal manufacturers
US $0.00/kg2025-11-28
- CAS:
- 4637-24-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $1.50/g2025-06-25
- CAS:
- 4637-24-5
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons