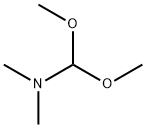
Application of N,N-Dimethylformamide dimethyl acetal
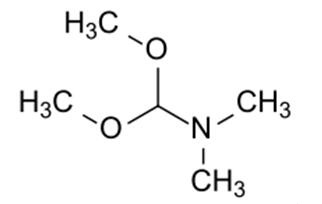
N,N-Dimethylformamide dimethyl acetal, also called dimethylformamid-dimethylacetal is a chemical compound with a molecular formula of C5H13NO2. It is a clear, colorless, amine-like smelling liquid that mixes with water and many organic solvents. In water, the acetal decomposes gradually [1]. The melting point is -85 °C. The boiling point is 102-103 °C (720 mm Hg(lit.)). The density is 0.897 g/mL at 25 °C(lit.). The refractive index is n20/D 1.396(lit.). The flash point is 45 °F.
N,N-dimethylformamide dimethyl acetal is an acetal obtained by formal condensation of N,N-dimethylformamide with methanol. N,N-dimethylformamide dimethyl acetal is a derivatisation agent used in gas-chromatography applications It has a role as a chromatographic reagent. It is an acetal and a tertiary amino compound. It derives from a N,N-dimethylformamide [2].
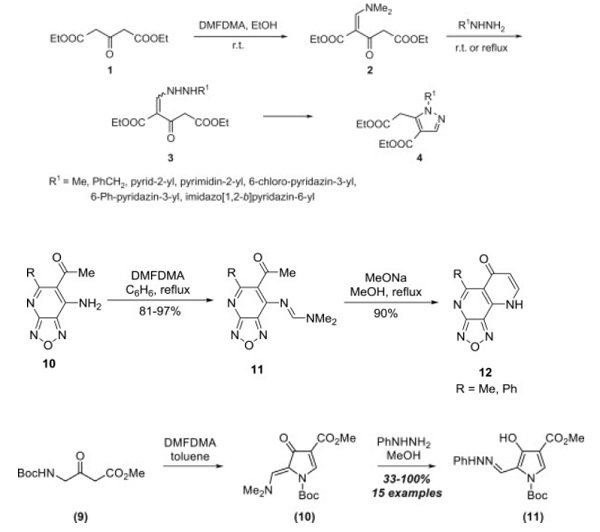
N,N-Dimethylformamide dimethyl acetal is the amide acetal formed by acetalizing dimethylformamide with methanol and bearing two alkoxy groups on the carbonyl group. The compound has become more widely used as a formylating reagent and methylating agent for carboxylic acids, phenols, amines, thiols and amino acids [1] and as a building block, in particular for heterocycles [3].
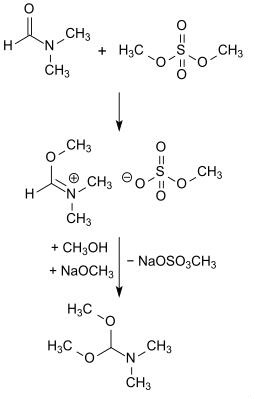
Analogous to the first publication on N,N-Dimethylformamide dimethyl acetal from the group of Hellmut Bredereck, N,N-Dimethylformamide dimethyl acetal of the obtained by reaction (O-methylation) of dimethylformamide with dimethyl sulfate adduct with sodium methoxide in methanol at 0 ° C in yields of 72 to 87 % received.
Because of the decomposition of the DMF-DMA in the distillation under atmospheric pressure, the rapid distillation of the reaction mixture with the addition of methanol as entrainer and subsequent fractional distillation of the resulting methanol / DMF (Dimethylformamide)-DMA (Dimethylacetamide) mixture is recommended. As a result, pure yields of N,N-Dimethylformamide dimethyl acetal of 85 to 90% are achieved.
The reaction of the Vilsmeier reagent N, N-dimethyl (chloromethylene) iminium chloride in chloroform with sodium methoxide in methanol affords DMF-DMA in 55% yield.
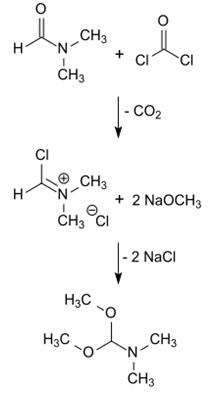
N,N-Dimethylformamide dimethyl acetal is also formed upon reaction of the reactants methanolate, DMF, and CHCl3. [15] Chloroform reacts with solid sodium methoxide or sodium methanolate in methanol, presumably via the intermediately formed dichlorocarbene: CCl2, which converts dimethylformamide to DMF-DMA with CO cleavage [4].
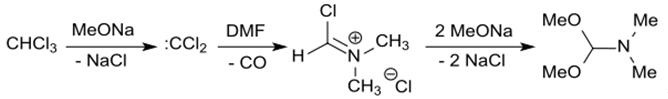
In millimolar approaches, crude yields are achieved up to 91%.
References
[1] U. Pindur: N,N-Dimethylformamide Diethyl Acetal. In: e-EROS Encyclopedia of Reagents for Organic Synthesis. 2001, doi:10.1002/047084289X.rd336.
[2] https://de.wikipedia.org/wiki/Dimethylformamid-dimethylacetal
[3] F.A. Abu-Shabab, S.M. Sherif, S.A.S. Mousa: Dimethylformamide dimethyl acetal as a building block in heterocyclic synthesis. In: J. Heterocyclic Chem. Band 46, Nr. 5, 2009, S. 801–827, doi:10.1002/jhet.69.
[4] P.L. Anelli, M. Brocchetta, D. Copez, D. Palano, M.V.P. Paoli: Unexpected formation of acylformamidines by reaction of primary carboxamides with MeONa in DMF in the presence of CHCl3. In: Tetrahedron. Band 53, Nr. 46, 1997, S. 15827–15832, doi:10.1016/0040-4020(97)10041-2
You may like
Related articles And Qustion
See also
Lastest Price from N,N-Dimethylformamide dimethyl acetal manufacturers
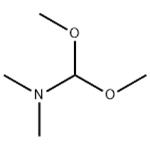
US $0.00/kg2025-11-28
- CAS:
- 4637-24-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
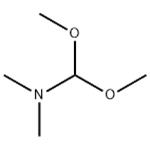
US $1.50/g2025-06-25
- CAS:
- 4637-24-5
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons