N,N-Dimethylformamide dimethyl acetal: Applications and Preparation
Introduction
N,N-Dimethylformamide dimethyl acetal (DMF-DMA) is an organic compound with the molecular formula C5H11NO2. It is a colorless liquid with a slightly sweet odor and is soluble in water and many organic solvents. DMF-DMA is commonly used as a reagent for organic synthesis, particularly as a protecting group for amines and as a carbonyl-activating agent for the formation of acetals and ketals. It can also be used as a solvent for various reactions, including Grignard reactions, and as a reagent for the reduction of carboxylic acids to aldehydes. In addition to its applications in organic synthesis, DMF-DMA has been used as a solvent and a stabilizer for certain polymers. It should be noted that DMF-DMA is flammable and may be toxic if ingested or inhaled, so it should be handled with care and appropriate safety precautions should be taken[1].

Figure 1 Appearance of N,N-Dimethylformamide dimethyl acetal
Applications
N,N-Dimethylformamide dimethyl acetal (DMF-DMA) is a useful reagent in organic synthesis with various applications. It can act as a solvent and electrophilic methyl group source in alkylation reactions.
DMF-DMA is commonly used in the synthesis of ketones and aldehydes, where it reacts with carboxylic acids to form an acylating agent that can be used to acylate nucleophiles such as alcohols, amines, and enolates. This method is particularly useful for synthesizing difficult-to-obtain sterically hindered ketones and aldehydes.
In addition to its role in synthetic chemistry, DMF-DMA is also employed as an intermediate in the production of pharmaceuticals, agrochemicals, and other fine chemicals. It serves as a building block for drugs like anti-inflammatory agents and antidiabetic drugs, as well as insecticides, herbicides, and fungicides. It has applications in polymer chemistry. It is used as a comonomer in the production of polyacrylonitrile fibers, which find use in textiles, filtration membranes, and carbon fiber production. DMF-DMA is also a solvent for the dissolution of various polymers and resins.
Overall, DMF-DMA is a versatile reagent with numerous applications in organic synthesis, pharmaceuticals, agrochemicals, and polymer chemistry[2-6].
Toxicity
N,N-Dimethylformamide dimethyl acetal, also known as DMF-DMA, is a chemical compound commonly used as a solvent in organic synthesis. However, it has been found to have toxic effects on humans and animals. Ingestion or inhalation of DMF-DMA can lead to respiratory distress, central nervous system depression, and liver damage. Prolonged exposure to this compound may also cause cancer and reproductive toxicity. Therefore, proper handling and disposal procedures should be followed when working with DMF-DMA to minimize the risk of exposure to its harmful effects.
Storage
When storing N,N-Dimethylformamide dimethyl acetal, it is important to follow certain guidelines to ensure its stability and prevent potential hazards. The compound should be kept in a cool, dry, and well-ventilated area, away from sources of heat, flames, and direct sunlight. It should also be stored in a tightly sealed container made of materials that are resistant to DMF-DMA's corrosive properties, such as glass or stainless steel. Proper labeling and handling procedures should be followed to avoid contact with skin, eyes, and clothing when handling the compound. In addition, it is recommended to store DMF-DMA separately from other chemicals to prevent contamination and accidental reactions. Regular monitoring and inspection of the storage area can help detect any signs of degradation or leakage, and proper disposal methods should be followed for any expired or damaged containers.
Preparation
N,N-Dimethylformamide dimethyl acetal (DMF-DMA) can be prepared by reacting N,N-dimethylformamide (DMF) with methanol in the presence of an acid catalyst. Here is a general procedure for the preparation of DMF-DMA:
In a 250 mL round-bottom flask, add 35 g (0.45 mol) of DMF and 45 mL of methanol. Add a catalytic amount of sulfuric acid (H2SO4) to the reaction mixture. The amount of H2SO4 required may vary depending on the reaction conditions, but typically ranges from 0.5% to 2% by weight of DMF. Stir the reaction mixture at room temperature or under reflux for several hours (typically 6-12 hours). The reaction progress can be monitored by TLC, which will show the disappearance of DMF and the appearance of DMF-DMA. Once the reaction is complete, cool the reaction mixture to room temperature and quench the reaction by adding a small amount of sodium bicarbonate (NaHCO3) to neutralize the acid catalyst. Filter the reaction mixture through a pad of celite to remove any solid impurities that may have formed during the reaction. Distill the crude DMF-DMA under reduced pressure to remove any residual solvent and unreacted starting materials. The product can be purified further if necessary using column chromatography or recrystallization.
It's important to note that DMF-DMA is a flammable and potentially toxic compound, and appropriate safety precautions should be taken when handling it.
References
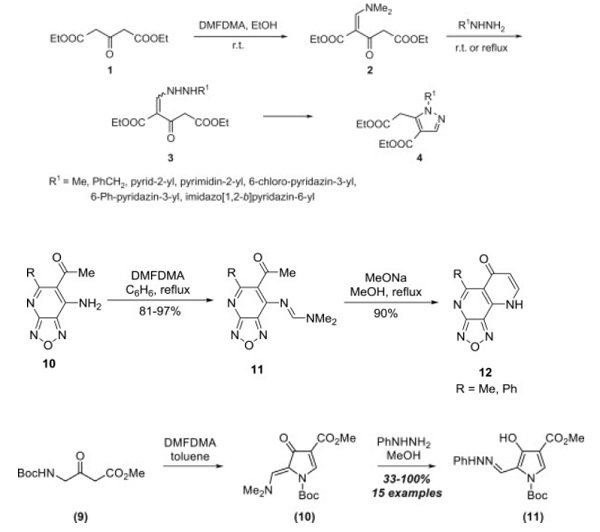
[1] Li X, Sun YX, Dai W, Liu W. Efficient synthesis and biological activity of 3,4-disubstituted pyridine-imidazole compounds using DMF-DMA as a reagent. Chinese Journal of Organic Chemistry, 2019; 39(6): 1560-1567.
[2] Liu Y, Wang J, Chen X. Synthesis of α-Amino Acids through Highly Stereoselective Additions of Grignard Reagents to N-(tert-Butoxycarbonyl) Imines Catalyzed by Scandium(III) Trifluoromethanesulfonate.[J] The Journal of Organic Chemistry. 2020; 85(2): 1222-1230.
[3] Wang H, Li Q, Luo Y, et al. Efficient Synthesis of 6-Substituted Benzimidazoles through a Tandem Reaction of o-Phenylenediamine with Aldehydes Using DMF-DMA as the Activating Agent.[J] RSC Advances.2018; 8(28): 15372-15378.
[4]Yu H, Jin L, Liu J, et al. A Novel Approach to Synthesize Bioactive Fused Pyrazolo[3,4-b]pyridines under Catalyst-Free Conditions.[J] Synthetic Communications. 2019; 49(13): 1634-1640.
[5] Zhang X, Feng Y, Li X,et al. Preparation of PAN Fibers and Their Characterization after Being Stabilized with Different Stabilizers.[J] Journal of Polymers and the Environment, 27(12), 2861-2870.
[6] Liu Z, Zhang Y, Bai L, et al. Synthesis of 1,3-Disubstituted Imidazolium Ionic Liquids under Microwave Irradiation and Their Catalytic Performances in Esterification Reactions.[J] Journal of Chemical Research. 2016; 40(5): 248-254.
Related articles And Qustion
Lastest Price from N,N-Dimethylformamide dimethyl acetal manufacturers
US $0.00/kg2025-11-28
- CAS:
- 4637-24-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise
US $1.50/g2025-06-25
- CAS:
- 4637-24-5
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons