The preparation method of iron chloride hexahydrate
Background
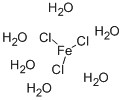
Iron chloride hexahydrate is a reddish-brown to yellow hexagonal crystal, odorless, astringent, and deliquescent. Molecular formula of Iron chloride hexahydrate: FeCl3.6H2O. Iron chloride hexahydrate can deliquescence into a reddish-brown liquid in the air. Iron chloride hexahydrate is very soluble in water, and the aqueous solution is strongly acidic, which can make proteins coagulate. Iron chloride hexahydrate is easily soluble in ethanol and acetone, but also in liquid sulfur dioxide, ethylamine and aniline, but insoluble in glycerol and phosphorus trichloride. The warehouse is low temperature, ventilated and dry; it is stored separately from strong oxidants and alkali metals. Iron chloride hexahydrate is widely used in water treatment, organic synthesis catalyst, and also used in dye and pharmaceutical industries. Iron chloride hexahydrate can be used as an analytical reagent for the determination of arsenic, lithium, tin, selenium, vanadium, thiocyanate, ferricyanate and serum total cholesterol, etc. It is industrially used for pigment and catalyst, etc. oxidizing agent[1].

Picture 1 Iron chloride hexahydrate crystal
Toxicological data
Acute toxicity of Iron chloride hexahydrate. Oral LDLo in rats: 900 mg/kg; intraperitoneal LC50 in mice: 260 mg/kg; LDLo injected in rabbits: 7200 ug/kg; oral LC50 in mice: 14700 mg/kg. Irritating to skin and eyes. Inhalation can cause sore throat, abdominal pain, diarrhea, nausea, etc. Staff should take precautions. After taking it by mistake, rinse your mouth with water and drink plenty of milk.
Ecological data
Generally slightly hazardous to water bodies, do not contact undiluted or large quantities of product with groundwater, water courses or sewage systems, and do not discharge material into the surrounding environment without government permission.
Preparation
Sand-like Iron chloride hexahydrate (FeCl3.6H2O) was prepared from anhydrous ferric chloride (FeCl3). Add 700 liters of pure water, 15 liters of hydrochloric acid, and 3800 kg of anhydrous ferric trichloride to a 1000-liter enamel-glass jacketed reaction pot, stir and dissolve, and filter with a centrifuge (canvas or 747 polypropylene filter cloth); then vacuum filter (747 polypropylene fiber) filter cloth) to measure the FeCl3 content. According to the ambient temperature, determine the batching coefficient Θ. The applicant has verified through a large amount of experiments that the batching coefficients Θ corresponding to ambient temperatures of 20°C, 15°C, 10°C, and 5°C are 0.9, L0, L1, and L2 respectively. According to the determined batching coefficient Θ, calculate the added amount of pure water, ensure that the Θ value is adjusted to between 0.9-1.20, cool under stirring, and let it separate out FeCl .6H O sandy crystal. Finally, centrifuge drying, packaging. The inner layer of the package is a polyethylene food packaging bag, the outer layer is a polyethylene bag with a thickness of 0.1mm, and the outermost layer is a cardboard drum.
The methods for industrial production of Iron chloride hexahydrate include hydrochloric acid method and one-step chlorination method. In the hydrochloric acid method, hydrochloric acid and washed iron filings are added to the reactor for reaction to generate ferric chloride solution. After clarification, chlorine gas is passed into the clear liquid for chlorination reaction to generate ferric chloride solution. Arsenic agent and heavy metal removal chemicalbook are used for solution purification, filtering to remove impurities, and then clarifying, cooling and crystallizing the clear liquid, solid-liquid separation and drying to obtain the finished product of Iron chloride hexahydrate, which is Fe+2HCl→FeCl2+H2O2FeCl2 +C12→2FeCl3
Use iron wire or iron powder to dissolve in hydrochloric acid below the calculated amount (heat if necessary). Insoluble matter was filtered off. Pour chlorine gas into the resulting aqueous solution of ferrous chloride until the smell of chlorine gas is emitted. Or oxidize with nitric acid, and then evaporate repeatedly with concentrated hydrochloric acid until the reaction of NO-3 is no longer obvious. At this time, the ferrous ions have been completely oxidized to ferric ions, and there is no need to check the residual ferrous ions [for example, use potassium hexacyanoferrate (III) potassium K3 [Fe(CN) 6] for the test. ]. Evaporate and concentrate the ferric chloride aqueous solution prepared above until the solution becomes solid after cooling. Due to the continuous loss of hydrogen chloride during the concentration process, it is necessary to frequently introduce hydrogen chloride.
Application
Iron chloride hexahydrate is mainly used as water treatment agent, corrosive agent in printing plate making, electronic circuit board, chlorinating agent in metallurgical industry, oxidant and mordant in dye industry, catalyst and oxidant in organic synthesis industry, chlorinating agent, It is the raw material for the manufacture of other iron salts and pigments and used in mine beneficiation. It is used as a flocculant in the purification of drinking water and industrial water supply. It has good solubility and excellent flocculation effect. Can be used for activated sludge dewatering. The pH range used is 6 to 11, and the optimum pH range is 6 to 8.4. The usual dosage is 5-100 mg/L. The flocs formed are coarse, the sedimentation speed is fast, and it is not affected by temperature. It is used to treat wastewater with high turbidity, and the effect is more significant. The corrosiveness of Iron chloride hexahydrate is stronger than that of ferrous sulfate, and the dosing equipment needs to be treated with anticorrosion.
When it dissolves in water, it produces hydrogen chloride gas, which pollutes the surrounding environment. In addition, Iron chloride hexahydrate can also be used as a waterproofing agent, a corrosive agent for printing and plate making, an oxidant and mordant in the dye industry, a catalyst for organic synthesis, and the manufacture of other iron salts. It can be used as etchant, catalyst, mordant, oxidant, chlorinating agent, condensing agent, disinfectant, hemostatic agent, feed additive, water purifying agent and analytical reagent, etc. Iron chloride hexahydrate is used in nutritional supplements (iron fortifiers). For infant milk powder, weaning food, etc.
Reference
1 1 Wang L, Li J, Zhao X, et al. An efficient and scalable room temperature aerobic alcohol oxidation catalyzed by iron chloride hexahydrate/mesoporous silica supported TEMPO[J]. Tetrahedron, 2013, 69(30): 6041-6045.
You may like
Related articles And Qustion
See also
Lastest Price from Iron chloride hexahydrate manufacturers

US $0.00/kg2025-09-19
- CAS:
- 10025-77-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $0.00/kg2025-05-15
- CAS:
- 10025-77-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg