Different applications of thioglycolic acid
Introduction
Thioglycolic acid is an organic acid with the chemical formula C2H4O2S. Toxic, colorless and transparent liquid with strong pungent odor. Miscible with water, soluble in ethanol, ether, soluble in common solvents. Thioglycolic acid is rapidly oxidized in the air, and in case of open fire and high heat, it burns and emits highly toxic hydrogen sulfide gas. It is a secondary organic acid corrosion product. [1] Thioglycolic acid is used to make epoxy resin, catalyst of bisphenol A. It is the main raw material of daily cosmetic cold iron and depilatory agent. It can also be used in the processing of PVC water pipes, water pumps and other products, food packaging, Equipment piping in a food processing plant. Thioglycolic acid is also a sensitive reagent for the determination of iron, molybdenum, lead, tin, etc.

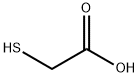
Picture 1 Thioglycolic acid liquid
Preparation
Thioglycolic acid can be obtained by reacting inorganic or organic sulfur-containing compounds with sodium and potassium salts of monochloroacetic acid. Such as: monochloroacetic acid reacts with sodium sulfide and sulfur to generate dithiodiacetic acid, which is then reduced with zinc and acid; or thiocarbamate reacts with monochloroacetic acid, and the product is obtained by hydrolysis; monochloroacetic acid and thiourea The reaction produces isothiourea acetic acid, which is then converted into precipitation with barium hydroxide, and then acidified with sulfuric acid to prepare an aqueous solution of thioglycolic acid, which can be evaporated to obtain a 60%-70% solution with a yield of over 70%. The reaction formula is: (HSCH2COO)2Ba+H2SO4→2HSCH2COOH+BaSO4
At a temperature not exceeding 25°C, add sodium carbonate solution to chloroacetic acid to pH 7-8, then add thiourea to react to generate acetic acid urea, and the obtained acetic acid thiourea reacts with sodium hydroxide at a temperature of 78-80°C After 2 hours, the reaction was cooled to 40°C, concentrated hydrochloric acid was added to the pH value of 3, and then extracted with ether. The ether layer obtained from the extraction was added to activated carbon and stirred, and then filtered. The filtrate was added with zinc powder and then rectified under reduced pressure to obtain pure sulfur. Glycolic acid. The process reaction formula is: ClCH2COOH+Na2CO3+S==C(NH2)2+NaOH→HSCH2COOH
Under stirring, 16% aqueous chloroacetic acid was mixed with 15% aqueous potassium hydrosulfide, ClCH2COOH:KHS=1:2 (molar ratio). Heating, adding a saturated solution of barium chloride in the same amount as chloroacetic acid, then adding 25% concentrated ammonia water, stirring evenly, standing, filtering, adding an equal volume of concentrated hydrochloric acid to the filtrate, then extracting with ether, and distilling off the ether , Distill at 2000Pa, collect 104~106℃ fractions, and obtain the finished product thioglycolic acid.
4. Sodium hydrosulfide and chloroacetic acid are prepared under the action of hydrogen sulfide and nitrogen.
The reaction formula is: NaSH+ClHCH2COOH→HSCH2COOH+NaCl [2]
Application fields
Thioglycolic acid is an intermediate in the production of thiomethoprol (caputril), biotin, thiozinc acid, sodium dithiosuccinate and other pharmaceuticals, and is also an intermediate in the synthesis of cysteine, hormonal agent, and industrial disinfectant. And an important raw material for the synthesis of sulfuric acid. Thioglycolic acid is used as antioxidant and stabilizer in pharmaceuticals to enhance the stability of the main drug and prolong the validity period of pharmaceutical preparations. Ammonium and sodium salts of thioglycolic acid are mainly used as curling agents, calcium salts can be used as depilatory agents, polymerization initiators, accelerators and chain transfer agents, and can be used for hair removal before cosmetic surgery and animal experiments. Thioglycolic acid is used to make epoxy resin, catalyst of bisphenol A, and it can also be used as the basic raw material for synthesizing PVC transparent plastic and organic antimony and organic tin heat stabilizer.
Thioglycolic acid is a sensitive reagent for the determination of iron, molybdenum, aluminum, tin, etc., and is an inhibitor of copper sulfide and iron sulfide minerals in beneficiation. In the petrochemical industry and the railway sector, it is used for cleaning and derusting of equipment and rails. It can be used as a crystallization nucleating agent in polypropylene processing and molding, as a modifier for coatings and fibers, as a blanket quickening agent, as a stabilizer raw material for polyvinyl chloride and rubber, as a cold perm agent, and as a pharmaceutical intermediate. Thioglycolic acid is used as a color developer for the photometric determination of molybdenum, rhenium and iron, and as a compounding masking agent.
Thioglycolic acid is used as a metal surface treatment agent. Adding a very small amount of thioglycolic acid can improve the cleaning effect of metal cleaning agents and immersion liquids, and can inhibit the corrosion of the treated metal surface, so it is often used as a rust inhibitor in the precision machining of metals. Thioglycolic acid is used as a low-toxicity or non-toxic stabilizer for polyoxyethylene. Octanoic acid thioglycolate, stannous methyl ester, octyl ester, 2-ethylhexyl ester, methoxybutyl ester, ethoxyethyl ester, etc. have low toxicity and non-toxicity. Approved by the U.S. Food and Drug Administration, the above esters It can be used as a low-toxic and non-toxic stabilizer. It can be used in the processing of PVC water pipes, water pumps and other products, food packaging, equipment pipes in food processing plants, and also in cosmetic factories and pharmaceutical factories, and this type of stabilizer improves product transparency and thermal stability.
Thioglycolic acid as a corrosion inhibitor for petroleum exploration. Thioglycolic acid can be used as an excellent corrosion inhibitor for olfactory salt completion fluids. When drilling high pressure wells in petroleum exploration, in order to protect oil and gas layers and reduce damage to formations, bromide-free solid phase completion fluids are required. Concentrated salt solutions are highly corrosive, and the corrosiveness increases with increasing temperature. The corrosion inhibitor that inhibits the corrosion of salt water at high temperature is only sulfur-containing compounds, and thioglycolate reacts with metal at a certain temperature to form a dense FeS protective film, which plays a role in corrosion inhibition.
Toxicological data
Thioglycolic acid is highly toxic. Its toxic effect may be related to the special effect of sulfhydryl group of some enzymes. The acute toxicity of thioglycolic acid is LD50<50mg/kg (oral in rats); 250mg/kg (oral in mice).
Ecological data
Biodegradability of thioglycolic acid. In the MITI-I test, the initial concentration was 100 ppm, the sludge concentration was 30 ppm, and the degradation was 100% after 4 weeks. Abiotic degradability of thioglycolic acid. In air, when the hydroxyl radical concentration is 5.00×106/cm4, the degradation half-life is 10 hours (theoretical).
Environmental standards
The maximum allowable concentration of harmful substances in the air of the former Soviet Union workshop: 0.1mg/m4[pi]
Hazardous properties
The routes of entry of thioglycolic acid are inhalation, ingestion, and absorption through the skin. The toxic effect of thioglycolic acid may be related to the special effect of sulfhydryl group of some enzymes. This product has a strong irritant. Eye contact can cause serious damage, resulting in permanent blindness. Can cause skin burns; sensitize the skin, causing allergic dermatitis. It can be absorbed through the skin and cause poisoning. Animal skin application of 10% solution of this product <5mL/kg will cause death. Thioglycolic acid may cause fire and explosion when exposed to open flame, high heat or contact with oxidants. Thermal decomposition produces toxic sulfide fumes. Has strong corrosiveness. Combustion (decomposition) products: carbon monoxide, carbon dioxide, sulfides.
Leak handling
Quickly evacuate personnel from the leaked contaminated area to a safe area, isolate them, and strictly restrict access. Cut off the source of ignition. It is recommended that emergency responders wear self-contained positive pressure breathing apparatus and acid-alkali-proof work clothes. Do not directly touch the leakage, cut off the source of leakage as much as possible, and prevent entry into restricted spaces such as sewers and flood drains. Small spills: Absorb or absorb with sand or other non-combustible materials. It can also be washed with plenty of water, diluted with the washing water and put into the waste water system. Large leakage: build embankments or dig pits for containment; cover with foam to reduce steam hazards. Transfer it to a tanker or a special collector with a pump, and recycle it or transport it to a waste disposal site for disposal.
Protective measures
Respiratory protection: Self-priming filter respirators (half masks) should be worn when exposure to its vapors is possible. It is recommended to wear self-contained breathing apparatus during emergency rescue or evacuation.
Eye Protection: Wear chemical safety goggles.
Protective clothing: Wear acid-alkali-proof overalls.
Hand Protection: Wear rubber acid and alkali resistant gloves.
Others: Smoking, eating and drinking are prohibited in the workplace, and hands should be washed before meals. After work, take a shower and change clothes. Store poison-contaminated clothes separately and wash them for later use. Practice good hygiene.
First-aid
Skin Contact: Remove contaminated clothing and rinse with plenty of running water for at least 15 minutes. seek medical attention.
Eye Contact: Immediately lift eyelids and rinse thoroughly with plenty of running water or saline for at least 15 minutes. seek medical attention.
Inhalation: Quickly leave the scene to fresh air. Keep the airway open. If breathing is difficult, give oxygen. If breathing stops, give artificial respiration immediately. seek medical attention.
Ingestion: If swallowed by mistake, rinse mouth with water and gastric lavage. Give milk or egg whites. seek medical attention.
Extinguishing method: Extinguishing agent: mist water, foam, sand.
Reference
1 Han Changri, Song Xiaoping. Production Technology Manual of Fine Organic Chemical Products Volume 2: China Petrochemical Press, 2010.06: 1624
Related articles And Qustion
See also
Lastest Price from Thioglycolic acid manufacturers

US $100.00/KG2025-04-21
- CAS:
- 68-11-1
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON

US $0.00/KG2025-03-21
- CAS:
- 68-11-1
- Min. Order:
- 200KG
- Purity:
- 99
- Supply Ability:
- 20000 kg