Understanding Ferric Chloride Hexahydrate: Comprehensive Insights
Introduction
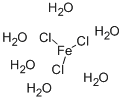
The molecular formula of iron chloride hexahydrate is FeCl3.6H2O. The compound is also called Ferric Chloride Hexahydrate. This inorganic chemical, recognized for its distinctive orange-brown crystals, is fundamental in water treatment and electronic manufacturing processes. Its efficacy in coagulating and flocculating in wastewater management transforms how contaminants are removed, making it indispensable in environmental engineering. It is widely utilized in chemical syntheses and analytical testing in laboratories, proving its versatility and essentiality in experimental and practical chemistry scenarios.
Chemical properties

It is a brown-red crystal that is odourless, astringent, has strong water absorption, is deliquescent, and can deliquesce into a reddish-brown liquid in the air. Ferric Chloride Hexahydrate is highly soluble in water, and the aqueous solution is strongly acidic, which can coagulate protein. The compound is also soluble in ethanol, acetone, sulfur dioxide liquid, ethylamine, and aniline but insoluble in glycerol and phosphorus trichloride.
Uses
Ferric Chloride Hexahydrate is mainly used as a water treatment agent, a corrosive agent for printing plate-making and electronic circuit boards, a chlorinating agent in the metallurgical industry, an oxidant and mordant in the dye industry, a catalyst and oxidant, and a chlorinating agent in the organic synthesis industry. It is a raw material used to make other iron salts and pigments and for mineral processing in mines[1].
It has also been increasingly utilized in various organic reactions, such as the oxidation of benzoin, an efficient catalyst for the Biginelli condensation, Michael reaction and esterification. A general and practical route for the moderate yield oxidative conversion of readily accessible 1,4-dihydropyridines (1,4- DHPs) to the corresponding pyridines was described using a relatively benign oxidant, i.e. ferric chloride hexahydrate ðFeCl3E6H2OÞ. The reaction was carried out under mild and convenient conditions. However, 4-isopropyl-1,4- DHP 1b oxidation with FeCl3E6H2O afforded the dealkylated pyridine[2].
References
[1] CN104003451A.pdf https://patentimages.storage.googleapis.com/11/e2/bb/82244b97d3b76a/CN104003451A.pdf
[2] Jun Lu. “FERRIC CHLORIDE HEXAHYDRATE: A CONVENIENT REAGENT FOR THE OXIDATION OF HANTZSCH 1,4-DIHYDROPYRIDINES.” Synthetic Communications 31 1 (2001): 2625–2630.
Related articles And Qustion
See also
Lastest Price from Iron chloride hexahydrate manufacturers

US $0.00/kg2025-09-19
- CAS:
- 10025-77-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $0.00/kg2025-05-15
- CAS:
- 10025-77-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg