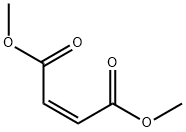
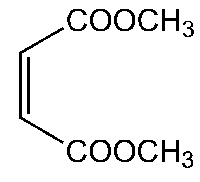
The application and production process of dimethyl maleate
Dimethyl maleate is an organic compound with the formula C6H8O4 and it is the dimethyl ester of maleic acid. Dimethyl maleate can be synthesized from maleic anhydride and methanol, with sulfuric acid acting as acid catalyst, via a nucleophilic acyl substitution for the monomethyl ester, followed by a Fischer esterification reaction for the dimethyl ester [1].
Applications
Dimethyl maleate acts as a dienophile involved in ultrasonic irradiation promoted Diels-Alder reaction with substituted furans. It is widely used as an intermediate and additive for pharmaceuticals, copolymers, pigments, plastics, paints, adhesives and agrochemicals. As an internal modifier, it increases the glass transition temperature of styrene or vinyl chloride polymer. It plays a vital role to improve the hardness and toughness of polymer films such as copolymers of vinyl acetate [2].
Dimethyl maleate is used in many organic syntheses as a dienophile for diene synthesis. It is used as an additive and intermediate for plastics, pigments, pharmaceuticals, and agricultural products. It is also an intermediate for the production of paints, adhesives, and copolymers. Dimethyl maleate has also found use in applications where improvements in the hardness and toughness of polymer films are desired. This includes, in particular, the improvement of anti-blocking properties of copolymers of vinyl acetate with DMM. It is also used as an internal modifier to increase the glass transition temperature of styrene or vinyl chloride polymers.
Production process
The production process of dimethyl maleate was described as below. It is well known that the production of an ester may be achieved by reacting a carboxylic acid with an alcohol in an acid catalyzed reversible reaction in which water is formed as a by-product. It is normally necessary to remove the so-called "water of esterification" to drive the equilibrium in favor of the ester. Further, since the presence of water will drive the equilibrium away from ester formation, any water present must be removed before esterification is completed. It is an improved esterification process which can successfully take place in the presence of water of the solution.
The process includes four steps (a) providing a feed solution comprising the maleic acid and the water of solution; (b) reacting the feed solution comprising maleic acid in an esterification zone with methanol to form dimethyl maleate and water of esterification; said esterification being conducted in a two-stage process, wherein the first stage is carried out in a first reactor in which the maleic acid is converted to a mono ester and the second stage is carried out in a second reactor in which the mono ester is converted to dimethyl maleate, the second reactor is a column reactor operated at a temperature of from 65 to 150°C and pressure from 1 to 5 bar (100 kPa to 500 kPa); (c) removing the water of solution and the water of esterification; and (d) recovering the dimethyl maleate [3].
reference
[1] https://en.wikipedia.org/wiki/Dimethyl_maleate
[2] https://www.alfa.com/en/catalog/A18944/
[3] David Mark Sutton, Graham Reed and Andrew George Hiles, Process for the production of dimethyl maleate, EP1678117B1
Related articles And Qustion
See also
Lastest Price from Dimethyl maleate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 624-48-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $50.00/kg2025-04-15
- CAS:
- 624-48-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000kg/week