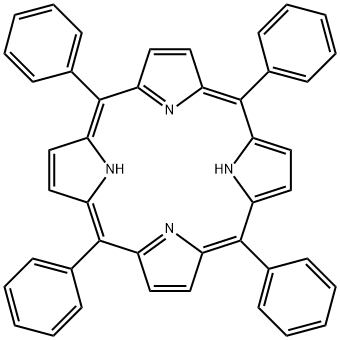
TPP - application on synthetic works
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents.
Tetraphenylporphyrin is hydrophobic, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform and benzene. Tetraphenylporphyrin is also an important organic intermediate to synthetize substituted porphyrin products
The following example is about its application on the synthesis of Ni porphyrins [1]
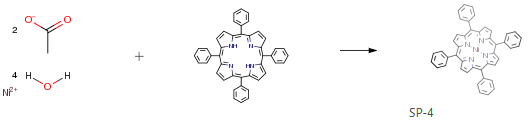
In a 250mL distillation flask, 5,10,15,20-tetraphenylporphyrin (H2TPP) (0.50g, 0.81mmol) and NaOAc (0.30g, 3.6mmol) was stirred in 75mL of chlorobenzene and 50mL of DMF. After the addition of two equivalents of metal acetate, a Soxhlet extractor with a cellulose filter thimble filled with ∼3g of K2CO3 was attached to the distillation flask. The assembly was completed with a condenser on the top of the extractor; and then the mixture was heated to reflux at 150°C overnight. The reaction extent was monitored by TLC or UV-Vis until all the H2TPP was consumed. After the reaction was compete, the solvent was removed under vacuum. The remaining solid was dissolved in 150mL of chloroform, and washed with water (50mL×3). The organic layer was further washed with a saturated sodium bicarbonate solution (50mL×3), and then dried over K2SO4. After removal of the solvent in vacuo, the solid was recrystallized from chloroform/heptane.
The following example is about its application on the synthesis ofβ-brominated tetraphenylporphyrins [2]
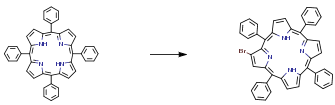
0.02 g (0.112 mmol) of N-bromosuccinimide was added to a solution of 0.04 g (0.0649 mmol) of staring material in 10 mL of DCM. The reaction mixture was refluxed at stirring during 45-60 min. 0.16 mL of pyridine was added after cooling, and the solvent was evaporated. The residue was washed with ethanol, dried, dissolved in a minimal amount of chloroform, and purified by chromatography on silica gel (eluent hexane-dichloromethane 1:1). Yield 0.034 g (75%,0.0495 mmol).
The following example is about its application on nitration of tetraphenylporphyrin [3]
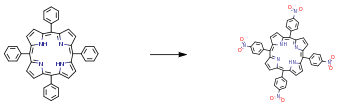
Under an argon atmosphere, red fuming HNO3 (2.4 mL, 56.7 mmol,) was added dropwise over a period of 20 min at 0 °C to a 150 mL chloroform solution of H2TPP (1.0 g, 1.62 mmol). The reaction was kept for 30 min and then quenched with an aqueous ammonia solution slowly. The organic layer was extracted and evaporated to dryness under reduced pressure. The residual brown powder was washed with boiling water and then subjected to a column separation process. In the process, the powder was first absorbed onto silica gel followed by an elution with ethyl acetate. The remained dark red band was collected and added into a KOH solution (5 percent w/v, molar ratio of KOH to silica gel) to remove silica gel. Centrifugation along with repetitive washing with water afforded the pure product in 87 percent yield (1.12 g).
References
1.Yao SA, Hansen CB, Berry JF. A convenient, high-yielding, chromatography-free method for the insertion of transition metal acetates into porphyrins[J]. Polyhedron, 2013, 58:2-6
2.Maltseva Z, Mamardashvili NZ. Complex formation of β-brominated tetraphenylporphyrins and metal exchange of their cadmium complexes with d-metal salts in dimethylformamide[J]. Russian Journal of General Chemistry, 2016, 86(1):102 – 109.
3.Xue Z, Lee PPS, Wang Y, Kwong DWJ, Li J, Xin JH, Wong WK, Cheuk KKL. Further insight into aryl[J]. Tetrahedron, 2011, 67(33):6030-6035.
See also
Lastest Price from TPP manufacturers

US $10.00-50.00/KG2025-06-18
- CAS:
- 917-23-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $10.00/KG2025-04-21
- CAS:
- 917-23-7
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt