Dimethyl Maleate: A Stable Compound with Safety Precautions for Handling and Storage
General Description
Dimethyl maleate, with the chemical formula C6H8O4, exhibits good stability under normal conditions and lacks known reactive hazards. It is essential to avoid incompatible materials such as acids, bases, and excessive heat to maintain its stability. While no hazardous reactions or polymerization occur during normal processing, decomposition may yield harmful products like CO and CO2. Classified as hazardous, precautions include protective gear, proper ventilation, and immediate medical attention if exposed. Strict storage and disposal practices are necessary to ensure safe handling. Overall, cautious handling adhering to safety protocols is crucial for minimizing risks associated with Dimethyl maleate.

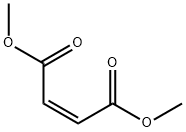
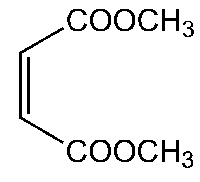
Figure 1. Dimethyl maleate
Structure
Dimethyl maleate (C6H8O4) is a chemical compound with a molecular weight of 144.12532 g/mol. Its IUPAC name is 1,4-dimethyl (2Z)-but-2-enedioate. The SMILES string representation of Dimethyl maleate is COC(=O)C=CC(=O)OC, and its InChI is InChI=1S/C6H8O4/c1-9-5(7)3-4-6(8)10-2/h3-4H,1-2H3/b4-3-. The structure of Dimethyl maleate consists of 17 bonds in total. Among these bonds, there are 9 non-hydrogen bonds, 3 multiple bonds, 4 rotatable bonds, and 3 double bonds. Additionally, it contains 2 aliphatic ester groups. In the 2D skeletal formula, the carbon atoms are represented at the corners, while hydrogen atoms attached to carbon atoms are not explicitly shown. Each carbon atom is assumed to be bonded to enough hydrogen atoms to fulfill its tetravalency. To visualize the spatial arrangement of atoms and bonds, a 3D model of Dimethyl maleate using the ball-and-stick representation is employed. In this model, the spheres representing atoms have a smaller radius compared to the bond lengths, enabling a clearer view of the molecular structure. This 3D model provides a comprehensive representation illustrating both the three-dimensional positions of the atoms and the bonds connecting them in Dimethyl maleate. 1
Applications
Dimethyl maleate demonstrates good stability and reactivity characteristics. It is considered stable under normal conditions with no known reactive hazards. To maintain its stability, it is essential to avoid contact with incompatible products and exposure to excess heat. Incompatible materials include acids, bases, reducing agents, and oxidizing agents, which should be kept separate from Dimethyl maleate to prevent any adverse reactions. In the event of decomposition, hazardous products such as carbon monoxide (CO) and carbon dioxide (CO2) may be released. However, under normal processing conditions, there are no hazardous reactions or polymerization processes associated with Dimethyl maleate. Overall, proper handling and storage practices should be followed to ensure the continued stability of the chemical and minimize any potential risks of reactivity. 2
Precautions and Storage
Dimethyl maleate is classified as a hazardous chemical according to the 2012 OSHA Hazard Communication Standard. It is labeled with the signal word "Warning" and is associated with several hazard statements, including harmful if swallowed, may cause allergic skin reaction, serious eye irritation, respiratory irritation, and damage to organs through prolonged or repeated exposure. Precautionary measures include thoroughly washing face, hands, and exposed skin after handling, refraining from eating, drinking, or smoking while using the product, and avoiding inhalation of dust, fumes, gas, mist, vapors, or spray. It is recommended to use the chemical only outdoors or in a well-ventilated area and to wear protective gloves, clothing, eye protection, and face protection. In case of exposure, individuals should seek medical attention if feeling unwell, remove victims to fresh air if inhaled, wash affected skin with plenty of soap and water, rinse eyes cautiously with water for several minutes, and call a poison center or doctor if swallowed. The chemical should be stored in a well-ventilated place with containers tightly closed and disposed of at an approved waste disposal plant. Additionally, engineering measures should be implemented to ensure adequate ventilation, eyewash stations, and safety showers close to the workstation. Personal protective equipment, such as protective eyeglasses, gloves, and clothing, should be worn to prevent skin exposure. Accidental release should be managed by using inert absorbent material and keeping the substance in suitable, closed containers for disposal. Overall, handling of dimethyl maleate should be carried out with caution, adhering to strict safety measures and environmental precautions to minimize potential risks and ensure safe usage and storage. 3
Reference
1. Structure & Deep Data of Dimethyl maleate (C6H8O4). Chemical Compound Deep Data Source.
2. SAFETY DATA SHEET: Dimethyl maleate. Thermo Fisher SCIENTIFIC. 2021, Cat No.: AC218460000.
3. Dimethyl maleate. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 5271565.
Related articles And Qustion
Lastest Price from Dimethyl maleate manufacturers

US $10.00/KG2025-04-21
- CAS:
- 624-48-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $50.00/kg2025-04-15
- CAS:
- 624-48-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000kg/week