Stanolone: biochemistry, physiology, and clinical implications
General Description
Stanolone, synthesized primarily through the action of microsomal SRD5A on testosterone, plays a crucial physiological role. It binds to SHBG and albumin in the bloodstream, with higher affinity to SHBG than testosterone. Metabolized by enzymes like 3α-HSD and 3β-HSD, it undergoes extensive metabolism in peripheral tissues. UGT facilitates the elimination of its metabolites. Understanding Stanolone's biochemistry is essential for medical treatments and risks associated with performance-enhancing drugs. Clinical implications include the treatment of androgen deficiency disorders, hormone-sensitive breast cancer, and androgenetic alopecia. Stanolone acts as a supplement in hormonal imbalances, inhibits tumor progression by competing with estrogen receptors, and stimulates hair growth while preventing hair loss. Overall, Stanolone has diverse clinical applications and is valuable in promoting normal development, inhibiting cancer growth, and addressing hair loss.

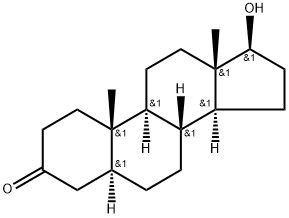
Figure 1. Stanolone
Biochemistry
Stanolone is synthesized primarily via the irreversible action of microsomal SRD5A (both types I and II) on testosterone. This process follows Michaelis-Menton kinetics and is not affected by age. SRD5As are localized in various tissues, including the prostate, skin, liver, and hair follicles, and catalyze the formation of Stanolone from testosterone in these tissues. Polymorphisms in the 5α-reductase type 2 (SRD5A2) gene have been hypothesized to increase the risk of prostate cancer by increasing 5α-reductase activity and Stanolone concentrations in the prostate. However, little Stanolone synthesized in the prostate or liver enters the general circulation due to efficient intracellular mechanisms that initially metabolize Stanolone into androstanediol and have little androgen activity. Additionally, Stanolone can be synthesized in tissues by "backdoor pathways" that enable its formation in the absence of testosterone or androstenedione as precursors. Overall, understanding the biochemistry of Stanolone is important for both medical treatments for conditions like hypogonadism and the potential risks associated with the abuse of performance-enhancing drugs. 1
Physiology
Stanolone is an androgen hormone that plays a crucial role in the body's physiology. It primarily binds to sex hormone-binding globulin (SHBG) and albumin in the bloodstream, with higher binding affinity to SHBG compared to testosterone. SHBG-Stanolone complexes can activate downstream events but do not bind directly to the SHBG receptor. Stanolone undergoes extensive metabolism in peripheral tissues by enzymes like 3α-hydroxysteroid dehydrogenase (3α-HSD) and 3β-hydroxysteroid dehydrogenase (3β-HSD). The liver, intestine, skin, and androgen-sensitive tissues contribute to metabolizing Stanolone into inactive steroids. UDP-glucuronyltransferase (UGT) facilitates the elimination of Stanolone metabolites through urine and bile. Different tissues have varying expression of UGT isozymes, which helps regulate intracellular androgen concentrations. Polymorphisms in genes responsible for androgen metabolism, such as UGT2B15 and UGT2B17, may impact prostate cancer risk and disturb Stanolone concentration regulation. Metabolic clearance is an important factor influencing the residence time of Stanolone in the body. Stanolone's metabolic clearance is approximately 70% that of testosterone. Adipose tissue plays a significant role in Stanolone metabolism, while muscle has minimal conversion of testosterone to Stanolone. The site of administration, such as intravenous or transdermal, affects the specific metabolites produced. In conclusion, understanding Stanolone's complex physiology, including protein binding, metabolism, and metabolic clearance, is essential for comprehending its effects and regulation in the body. 2
Clinical implications
Stanolone is a potent androgen hormone that plays a crucial role in the development and maintenance of male characteristics. It is primarily produced in the testes and acts on various target tissues throughout the body. Clinical implications of Stanolone are diverse and significant. One notable application is in the treatment of androgen deficiency disorders. In cases where individuals have insufficient levels of naturally occurring androgens, such as in hypogonadism or delayed puberty, exogenous Stanolone can be administered to supplement the hormonal imbalance and promote normal development and sexual maturation. Stanolone's androgenic properties make it valuable in managing certain medical conditions, including breast cancer. In hormone-sensitive breast cancer, where tumor growth is stimulated by estrogen, Stanolone can be utilized as an anti-estrogenic agent to inhibit tumor progression. It acts by competing with estrogen receptors, reducing the availability of estrogen and subsequently impeding cancer cell proliferation. Additionally, Stanolone has been implicated in the treatment of male pattern baldness (androgenetic alopecia). Its ability to bind to androgen receptors in the scalp follicles helps stimulate hair growth and prevent further hair loss, making it a potential therapeutic option for individuals experiencing this condition. In conclusion, Stanolone has clinical implications in the management of androgen deficiency disorders, hormone-sensitive breast cancer, and androgenetic alopecia. Its androgenic properties and interactions with specific receptors make it a valuable tool in promoting normal development, inhibiting cancer growth, and addressing hair loss. 3
Reference
1. Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, LaValley MP, Mazer NA, Bhasin S. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab. 2010 Aug;95(8):3955-3964.
2. Marchetti PM, Barth JH. Clinical biochemistry of dihydrotestosterone. Ann Clin Biochem. 2013 Mar;50(Pt 2):95-107.
3. Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA. Dihydrotestosterone: Biochemistry, Physiology, and Clinical Implications of Elevated Blood Levels. Endocr Rev. 2017 Jun 1;38(3):220-254.
You may like
Related articles And Qustion
See also
Lastest Price from Stanolone manufacturers

US $10.00/box2025-10-12
- CAS:
- 521-18-6
- Min. Order:
- 1box
- Purity:
- 99.99%
- Supply Ability:
- 100000box

US $0.00/box2025-09-26
- CAS:
- 521-18-6
- Min. Order:
- 1box
- Purity:
- 0.99
- Supply Ability:
- 10tons