The applications and side effects of Entecavir
Description
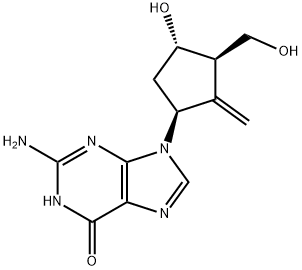
Entecavir is an organic compound with the chemical formula C12H15N5O3. It is known chemically as 2-amino-1, 9- dihydro- 9- [(1S, 3R, 4S) -4- hydroxy- 3- (hydroxymethyl)- 2- methylenecyclopentyl]-6H-purin-6-one. As an analog of 2′-deoxyguanosine, Entecavir has potent activity against HBV replication.
Applications
Entecavir is approved for the treatment of hepatitis B infection with compensated or decompensated liver disease in adults with evidence of active viral replication and either evidence of persistent transaminase elevations or histologically active disease. It belongs to the family of medicines called antivirals. Antivirals are used to treat infections that are caused by viruses. This medicine will not cure the hepatitis B virus, but it will keep it from reproducing and causing more liver damage. Entecavir can also be used for patients with lamivudine-resistant viremia. Adjustment in dosing should be considered for patients with CrCl < 50 mL/min. Entecavir triphosphate inhibits HBV polymerase base priming, reverse transcription of the negative strand from the pregenomic messenger RNA, and synthesis of positive-strand HBV DNA. It is approximately 30-fold more potent than lamivudine in vitro.232 Entecavir triphosphate is a weak inhibitor of cellular DNA polymerases, and it does not demonstrate activity against HIV-1 replication.
Mechanism of action
Entecavir affects three steps in the replication of HBV: (1) prevents the priming of the HBV reverse transcriptase, (2) prevents reverse transcribing of the HBV pregenomic mRNA, and (3) inhibits DNA-dependent DNA synthesis (i.e., terminating viral DNA synthesis). The HBV polymerase binds preferentially to entecavir triphosphate, and entecavir triphosphate does not affect human mitochondrial DNA synthesis.
Side effects
The adverse effect profile of entecavir is similar to lamivudine, including the same black box warnings. Common CNS effects include pyrexia (14% with decompensated liver disease), headache (2–4%), fatigue (1–3%), and dizziness. In preclinical animal trials, there was a higher incidence of solid tumors with high-dose, prolonged administration of entecavir. Studies are ongoing to evaluate the long-term treatment effects of entecavir.
References
[1] Ahrens, Christine L. , and E. M. Manno . "Neurotoxicity of commonly used hepatic drugs. " Elsevier(2014).
[2] Said, Adnan , N. Safdar , and M. R. Lucey . Liver Disease Among Renal Transplant Recipients. 2019.
[3] Benhamou, and Yves. "Hepatitis B in the HIV-Coinfected Patient."JAIDS Journal of Acquired Immune Deficiency Syndromes 45.Supplement 2(2007):S57-S65.
Related articles And Qustion
See also
Lastest Price from Entecavir manufacturers

US $0.00/kg2025-11-27
- CAS:
- 142217-69-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/g2025-04-21
- CAS:
- 142217-69-4
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000g