Entecavir: Preparation, Pharmacodynamics, Safety and Chemical Studies
General Description
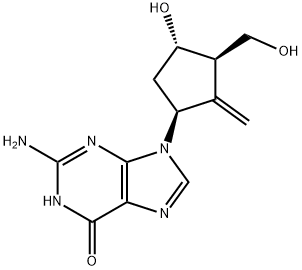
The entecavir, a viral replication inhibitor[1],and belongs to a carbocyclic guanosine nucleoside analog with potent selectiity against hepatitis B virus because of low toxicity and acted through inhibition of DNA polymerase. Therefore, it was approved for the treatment of chronic HBV infection in adults.Additionally,The entecavir has remarkable potency, resistance, and safety profile.[2]

Figure 1 Entecavir powder
Preparation
One novel synthesis of Entecavir that used the intramolecular nitrile oxide cycloaddition (INOC) reaction to construct carbocyclic core and a Mitsunobu reaction to install the purine base.
Method 1.The retrosynthetic route to Entecavir is illustrated in the following text. For the stereoselective introduction of the purine base, we planned to perform a Mitsunobu reaction to allyl alcohol of compound 2, the product of a diastereoselective reduction of 3. Enone 3 could be formed by intramolecular nitrile oxide cycloaddition reaction of 4, followed by hydrogenolysis of isoxazoline ring and dehydration of the resulting alcohol. The required precursor 4 can in turn be synthesized from epoxide 5, available from 1,3-propanediol 6.
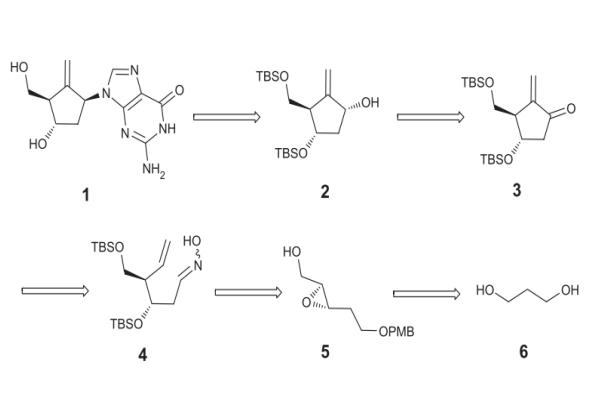
Figure 2 Retrosynthetic analysis of entecavir from 1,3-propanediol.
Method 2. Preparing the substrate for INOC reaction started with selective protection of 1,3-propanediol 6 with p-methoxybenzyl chloride. The resulting mono-protected diol 7 was oxidized to the corresponding aldehyde by Swern oxidation, followed by a Wittig olefination to give compound 8 in a 90% yield (two steps from 6).
Reduction of the ester residue in 8 with DIBAL-H and the subsequent Sharpless epoxidation of the resulting allylic alcohol gave epoxide 5 in an 88% yield with an enantioselectivity of>98%. Ring opening of 5 with vinylmagnesium bromide in the presence of cuprous iodide led to the desired diol 9 in an 84% yield. Protection of the hydroxyl group with TBSCl and imidazole in DMF and removal of PMB group with DDQ delivered alcohol 10 in a 90% yield. Swern oxidation of compound 10, followed by treatment with hydroxylamine hydrochloride and NaOAc in MeOH and exposure of the resulting oxime to sodium hypochlorite in methylene chloride provided the isoxazoline 11 and 12 in 6% and 76% yields, respectively, over three steps. The relative stereochemistry of the major isomers 12 was assigned by carrying out NOE analysis. Hydrogenolysis/hydrolysis of 11 and 12 with H2 (1 atm) and 10% Pd/C or W-2 Raney nickel in aqueous THF containing B(OH)3 gave the hydroxyl ketone, which, without purification, was treated with MsCl and Et3NinCH2Cl2, providing the enone 3 in a 76% yield over two steps. In order to execute the planned Mitsunobu reaction, the enone group needed to be reduced stereoselectively. Among the reducing agent we screened, reduction of enone with LiBH(Et)3 was found to be superior to other conditions tested and provided the desired allylic alcohol 2 in a 90% yield with a small amount of b-hydroxyl diastereomer (6%). Treatment of 2 with DEAD and PPh3 in THF produced the compound 13 in an 80% yield, which upon treatment with concd HCl gave entecavir 1 in a 96% yield.[2]
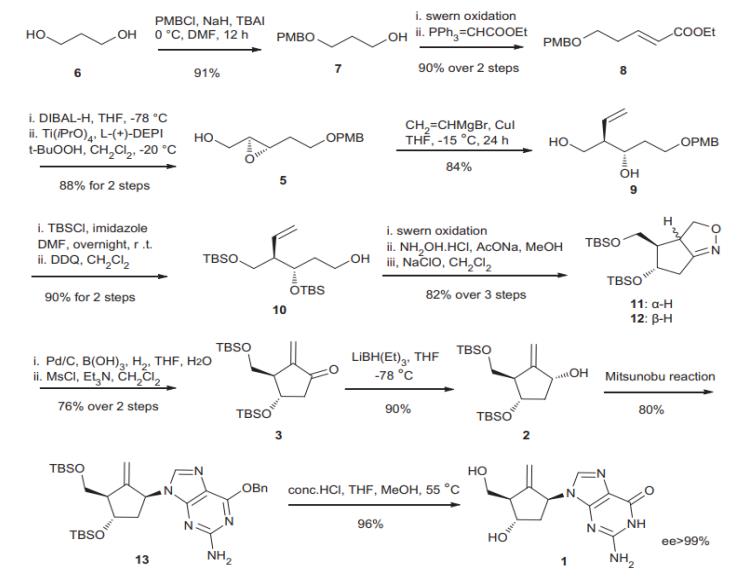
Figure 3 Synthesis of Entecavir (1).
Pharmacodynamics
Entecavir has a EC50 of 3.75 nM against HBV that is incorporated into the protein primer of HBV and subsequently inhibits the priming step of the reverse transcriptase. The antiviral activity of entecavir is significantly less against the other RNA and DNA viruses[4]. The intracellular half-life of entecaviris 15h[5].Daily oral treatment with entecavir at doses ranging from 0.02 to 0.5mg/kg of body weight for 1 to 3 months is effective in ;reducing the level of woodchuck hepatitis virus (WHV) viremia in chronically infecte woodchucks[6].
Application
The entecavir has demonstrated effective antiviral suppression and good tolerability in Chinese patients with CHB. In this patient population, entecavir has shown antiviral efficacy comparable with that of other recommended nucleos(t)ide therapies, including lamivudine and adefovir, with a number of studies demonstrating superior efficacy with entecavir. Entecavir reduces the risk of hepatic events in Chinese patients with CHB, and has demonstrated efficacy in those with HBV-associated decompensated cirrhosis and liver failure. In addition, entecavir can prevent HBV reactivation in patients undergoing immunosuppressive therapy.[7]The entecavir may be an effective treatment for HBV‑related cirrhosis via reducing HBV DNA viral load, increasing liver function and activating the complement system. Based on the results of the present study, entecavir appears to be a more effective treatment for patients with compensated cirrhosis compared with patients with decompensated cirrhosis.[8]
Safety
Long-term use was reported to be associated with a very low rate of side effects. Adverse events were not dose-related; their frequencies were similar between 0.5 or 1 mg doses of entecavir. The most frequent adverse events in clinical trials were headache, upper respiratory tract infection, cough, nasopharyngitis, fatigue, dizziness, upper abdominal pain and nausea. Most of these adverse effects were mild or moderate severity and did not require discontinuation of the drug. Severe adverse events accounted for 7%-10% and discontinuation of therapy accounted for 1%-2% of patients.
Entecavir is a good option for the treatment of CHB patients with decompensated cirrhosis because of the rapid effect on HBV decline and low resistance rates.Entecavir has been reported to have a high safety profile in decompensated patients. Nevertheless, the patients should be monitored cautiously for the risk of lactic acidosis during the treatment and entecavir should be suspended in the case of suspected lactic acidosis.Patients with severe acidosis complained of nausea, dyspnea and weakness, and showed a reduced general physical condition, impaired consciousness and tachypnea.Therefore, long-term use of entecavir is generally safe and associated with low rates of serious adverse events, and discontinuation of the treatment is rarely required. ALT flares were low in patients receiving entecavir and generally associated with the improvement of liver disease. In current guidelines, entecavir is also recommended as treatment and prophylaxis of CHB infection in patients with renal transplant due to being an agent without signs of nephrotoxicity.No increased incidence of myopathy was reported with entecavir treatment, compared to placebo. Entecavir and lamivudine are not generally associated with renal adverse events.Although entecavir is suggested to be associated with lactic acidosis in CHB patients with high MELD scores, its use in compensated and decompensated cirrhotic patients were reported to be safe.[3]
Reference
[1]Billich A. Entecavir (Bristol-Myers Squibb)[J]. Current opinion in investigational drugs (London, England: 2000), 2001, 2(5): 617-621.
[2]Zhou B, Li Y. Synthesis of entecavir (BMS-200475)[J]. Tetrahedron Letters, 2012, 53(5): 502-504.
[3]Kayaaslan B, Guner R. Adverse effects of oral antiviral therapy in chronic hepatitis B[J]. World journal of hepatology, 2017, 9(5): 227.
[4]Innaimo SF, et al. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus[J]. Antimicrob Agents Chemother. 1997,41(7):1444-9.
[5]Rivkin A, et al. A review of entecavir in the treatment of chronic hepatitis B infection[J]. Curr Med Res Opin.2005,21(11):1845-57.
[6]Genovesi EV, et al. Efficacy of the carbocyclic 2'-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection[J]. Antimicrob Agents Chemother.1998,42(12):3209-18.
[7]Wang, J. Clinical utility of entecavir for chronic hepatitis B in chinese patients[J].Drug Design, Development and Therapy.2014,8, 13-24.
[8]Gai X D,et al. Effect of entecavir in the treatment of patients with hepatitis B virusrelated compensated and decompensated cirrhosis[J]. Experimental and therapeutic medicine.2017, 14(4): 3908-3914.
You may like
Related articles And Qustion
Lastest Price from Entecavir manufacturers

US $0.00/kg2025-11-27
- CAS:
- 142217-69-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/g2025-04-21
- CAS:
- 142217-69-4
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000g