Stannous sulfate: Preparation Method by Tin Powderization Waste, Applications as Electrolyte Additive and Nano-filler
General Description
The synthesis of stannous sulfate from tin powderization waste involves a two-step process, starting with the conversion of tin powder to stannous chloride using hydrochloric acid, followed by reacting the produced SnCl2 with ammonium sulfate to form stannous sulfate. Stannous sulfate has significant applications, including as an electrolyte additive in lead-acid batteries, where it enhances battery performance by reducing lead sulfate crystal size, and as a nano-filler in polyvinylidene fluoride nanocomposites, improving their phase transformation and material properties for advanced applications in smart sensing devices.

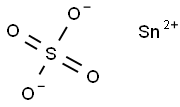
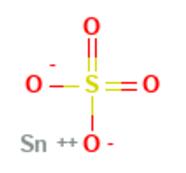
Figure 1. Stannous sulfate
Preparation method by tin powderization waste
The necessity of finding a way to utilize Sn metal from tin powderization waste as effectively and efficiently as possible has risen because of the large number of industrial by-products of Sn waste and the broad applications of tin chemicals in the world. Stannous chloride (SnCl2) and stannous sulfate (SnSO4) are tin-derived compounds in which their applications are in various fields, and one of which is catalysts. Catalyst products produced from the two compounds are STO (Sulfated Tin Oxide) catalyst. In one study from M. Nasikin and colleagues, tin powderization waste was used as raw material for the synthesis of SnCl2 and SnSO4. The purpose of using tin waste is an effort to create the Sustainable Development Goals (SDG) program launched by the United Nations to obtain a sustainable consumption and production system. Tin powder from the off-spec product of the tin powderization process can be used as raw material to manufacture tin-derived chemical compounds. Overall, the process of developing tin products will produce a non-waste system (zero waste). The optimum conditions to synthesize SnCl2 are as follows; the tin powder particle size is 500 mesh with HCl 12 M at 80°C with a yield percentage of 95%. The synthesis of SnSO4 with the reaction of SnCl2 + (NH4)2SO4 can be carried out using stirring techniques. The results of the FT-IR spectrometer showed a spectrum of sulfate groups in the region ~ 1181 cm−1.1
Applications
Applications as electrolyte additive
The effects of stannous sulfate(SnSO4) as an electrolyte additive on the microstructure of positive plate and electrochemical performance of lead acid battery made from a novel leady oxide are investigated by R. Vasant Kumar and colleagues. The novel leady oxide is synthesized through leaching of spent lead paste in citric acid solution. The novel leady oxides are used to prepare working electrode (WE) subjected to electrochemical cyclic voltammetry (CV) tests. Moreover, the novel leady oxides are used as active materials of positive plate assembled as a testing battery of 1.85 A h capacity. In CV tests, SEM/EDX results show that the major crystalline phase of the paste in WE after CV cycles is PbSO4. The larger column-shaped PbSO4 crystals easily generate in the paste of WE without an electrolyte additive of SnSO4. However, PbSO4 crystals significantly become smaller with the addition of SnSO4 in the electrolyte. In batteries testing, SEM results show that an electrolyte additive of SnSO4 could effectively decrease PbO2 particle size in the positive active materials of the teardown battery at the end of charging procedure. It is indicated that an electrolyte additive of SnSO4 could have a positive influence on restraining larger particles of irreversible sulfation in charge/discharge cycles of battery testing.2
Applications as nano-filler
Nanocomposites of PVDF-tin sulphate were synthesized by solution-mixing technique with 0–4% w/w loadings of stannous sulfate nanoparticles. The novel nanoparticles of SnSO4 were synthesized via in situ deposition method and its surface modification was performed by nonionic polymeric surfactant PEG. XRD analysis revealed that the average diameter of synthesized nanoparticles was 45 nm. Polymeric films of PVDF/SnSO4 nanocomposites were characterized by FTIR, SEM, EDS, TG/DTA, DSC and UV–vis spectrum. FTIR absorption bands show that α and β-phases coexist in pure PVDF which was supported by the 2θ value of XRD at 20.75°. This critical data shows that the phase of PVDF was completely transformed from α to β with incorporation of stannous sulfate nanoparticles. The UV absorbance intensity was increased, T g and T d were decreased with the increase in nanoparticles loadings in polymer matrix. The prepared nanocomposites could be used as a smart material for sensing device. 3
References:
[1] C. A. TAJUDDIN. Stannous chloride (SnCl2) and stannous sulfate (SnSO4) synthesis from tin powderization waste[J]. IOP Conference Series: Earth and Environmental Science, 2021, 171 1: 111341. DOI:10.1088/1755-1315/749/1/012025.[2] QIN WANG . Stannous sulfate as an electrolyte additive for lead acid battery made from a novel ultrafine leady oxide[J]. Journal of Power Sources, 2015, 285: 1-588. DOI:10.1016/j.jpowsour.2015.03.125.
[3] SONAM RATHORE G J Hari Madhav. Efficient nano-filler for the phase transformation in polyvinylidene fluoride nanocomposites by using nanoparticles of stannous sulfate[J]. Materials Research Innovations, 2019, 1 1: 50-90. DOI:10.1080/14328917.2017.1406572.
You may like
Related articles And Qustion
See also
Lastest Price from Stannous sulfate manufacturers

US $9.90/KG2025-04-21
- CAS:
- 7488-55-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $180.00/kg2025-04-15
- CAS:
- 7488-55-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton