Stannous sulfate: properties, applications and toxicity
General Description
Stannous sulfate is a versatile compound with important properties. It is a white solid soluble in water and acts as a reducing agent. With a low melting point and high boiling point, it is easy to handle and suitable for high-temperature applications. Stannous sulfate is used in electroplating, enamel production, and as a catalyst in organic synthesis. It can also be added to lead acid batteries to improve performance and reduce water loss. However, stannous sulfate can be toxic at high concentrations, causing respiratory, eye, and skin irritation, as well as potential organ damage. Proper safety precautions should be taken when handling this compound.

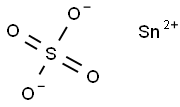
Figure 1. Stannous sulfate
Properties
Stannous sulfate, also known as tin(II) sulfate, is a chemical compound with the formula SnSO4. It is a white solid that is soluble in water and exhibits some important properties. Firstly, stannous sulfate is a reducing agent, meaning that it can donate electrons to other compounds. This property makes it useful in certain industrial processes, such as electroplating and the production of enamels and glazes. Secondly, stannous sulfate has a relatively low melting point of around 360°C, which makes it easy to handle and process. It also has a high boiling point of around 620°C, allowing it to be used in high-temperature applications. Finally, stannous sulfate is a versatile compound that can react with a wide range of other substances, forming various products. It is commonly used as a catalyst in organic synthesis and as a reagent in analytical chemistry. Overall, stannous sulfate is a valuable chemical compound with unique properties that make it useful in a variety of applications. 1
Applications
Stannous sulfate has been shown to be an effective electrolyte additive for improving the performance of deep cycle lead acid batteries. Research conducted by Ang demonstrated that the addition of Stannous sulfate can significantly enhance battery charging acceptance and reduce water loss. Although the influence mechanism of Stannous sulfate on battery performance was not fully explored, Bhattacharya suggested that Sn2+ ions may be adsorbed onto the plate surface, changing the structure of the PbSO4 layer and improving corrosion resistance. Furthermore, Wei's study used cyclic-voltammetry to show that Sn2+ ions could be reduced to tin on the negative plates or oxidized to Sn4+ species on the positive plates, which benefits the operation of lead acid batteries. In addition to Stannous sulfate, other electrolyte additives have also been studied for their effects on lead acid battery performance. H3PO4 has been found to reduce discharge capacity dependence on charge/discharge rate and improve discharge capacity. Liu's study showed that moderate doses of Na2SO4 added to the electrolyte can improve battery capacity, charge acceptance, and reduce water loss, prolonging the cycle life of the battery. Chahmana's study investigated the effect of various ions containing Sn2+, Sb3+, Co2+, Mg2+ and Al3+ in electrolyte on the formation of PbO2. Some metal ions were found to enhance the proportion of PbO2 in the gel area, improving cohesiveness with other materials. Overall, electrolyte additives have a significant influence on the performance of lead acid batteries, especially on the transformation of PbSO4 into PbO2 in the active material of positive plate, which can lead to battery failure. However, further research is needed to better understand the precise mechanism by which these additives affect battery morphology and overall performance. 2
Toxicity
The toxicity of stannous sulfate depends on the dose and route of exposure. High concentrations of stannous sulfate inhalation or ingestion can result in acute toxicity. Stannous sulfate can cause respiratory system, eyes, and skin irritation. Ingestion may lead to nausea, vomiting, abdominal pain, and diarrhea. Prolonged or repeated exposure can cause potential organ damage, such as to the liver, kidneys, and reproductive system. Although limited evidence suggests that stannous sulfate may have carcinogenic properties, more research is needed. To minimize the risks, it is essential to follow proper safety guidelines and work practices, wear personal protective equipment, ensure proper ventilation, and avoid direct contact or inhalation of the compound. 3
Reference
1. Chemical Abstracts Service (CAS). (2023). CAS REGISTRY Number: 7488-55-3. Retrieved from https://www.cas.org/
2. Wang Q, Liu J, Yang D, et al. Stannous sulfate as an electrolyte additive for lead acid battery made from a novel ultrafine leady oxide. Journal of Power Sources, 2015, 285: 485-492.
3. Agency for Toxic Substances and Disease Registry (ATSDR). (2005). Toxicological Profile for Tin. Retrieved from https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=582&tid=108
Related articles And Qustion
Lastest Price from Stannous sulfate manufacturers

US $9.90/KG2025-04-21
- CAS:
- 7488-55-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $180.00/kg2025-04-15
- CAS:
- 7488-55-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton

![2698-41-1 Properties of [(2-Chlorophenyl)methylene]malononitrileapplications of [(2-Chlorophenyl)methylene]malononitrilesafety of [(2-Chlorophenyl)methylene]malononitrile](https://www.chemicalbook.com/NewsImg/2020-1-10/2020110131412842.jpg)
![2698-41-1 Properties of [(2-Chlorophenyl)methylene]malononitrileapplications of [(2-Chlorophenyl)methylene]malononitrilesafety of [(2-Chlorophenyl)methylene]malononitrile](/NewsImg/2023-10-01/6383177416056814323387027.jpg)