What is Stannous Sulfate?
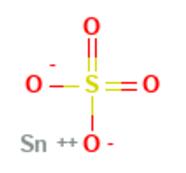
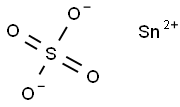
Fig 1. Chemical structure formula and three-dimensional structure of Stannous Sulfate
Stannous Sulfate is a white to slightly yellow, heavy crystalline powder. When dissolved in a 5% Sulfuric Acid Solution, a clear solution is obtained. Gradual hydrolysis occurs over a period of time with the formation of insoluble tin compounds.
Stannous Sulfate is commonly used in acid tin plating baths, liquor finishing and drawing of steel wire. It delivers a high current efficiency and smooth, fine grained deposits. A bright finish can be obtained by flow melting of through the use of certain additives.
Many stannate tin platers have converted to acid sulfate. Room temperature operation, expedited reaction, greater anode efficiency, and a bright finish or matte finish options may be listed among the advantages.
The inception of acid sulfate electroplating was in the electronics industry. Currently, however, any plater untroubled by the hydrogen embrittlement will utilize an acid sulfate process. Because the anode provides all the necessary tin, only a minimal quantity of Stannous Sulfate is requisite in order to maintain the plating bath. Thus, there is a profusion of companies of modest capacity with a requirement for electroplating Stannous Sulfate.
Stannous Sulfate is a chemical compound. It is a white solid that can absorb enough moisture from the air to become fully dissolved, forming an aqueous solution; this property is known as deliquescence. It can be prepared by a displacement reaction between metallic tin and copper(II) sulfate:
Sn (s) + CuSO4 (aq) → Cu (s) + SnSO4 (aq)
Stannous Sulfate is a convenient source of tin(II) ions uncontaminated by tin(IV) species.In the solid state the sulfate ions are linked together by O-Sn-O bridges. The tin atom has three oxygen atoms arranged pyramidally at 226 pm with the three O-Sn-O bond angles of 79°, 77.1° and 77.1°. Other Sn-O distances are longer ranging from 295 - 334pm[1-3].
Stannous Sulfate is a moderately water and acid soluble Tin source for uses compatible with sulfates. Sulfate compounds are salts or esters of sulfuric acid formed by replacing one or both of the hydrogens with a metal. Most metal sulfate compounds are readily soluble in water for uses such as water treatment, unlike fluorides and oxides which tend to be insoluble. Organometallic forms are soluble in organic solutions and sometimes in both aqueous and organic solutions. Metallic ions can also be dispersed utilizing suspended or coated nanoparticles and deposited utilizing sputtering targets and evaporation materials for uses such as solar cells and fuel cells. Stannous Sulfate is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered.
References
[1] Tin (inorganic compounds, as Sn)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
[2] Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 451.
[3] Donaldson, J. D.; Puxley, D. C. (1972). "The crystal structure of tin(II) sulphate". Acta Crystallographica Section B. 28 (3): 864–867.
[4] https://pubchem.ncbi.nlm.nih.gov/compound/62643
[5] http://www.chemspider.com/Chemical-Structure.56395.html?rid=54d368d1-05ff-4e99-8877-823dfe93759c
You may like
Related articles And Qustion
See also
Lastest Price from Stannous sulfate manufacturers

US $9.90/KG2025-04-21
- CAS:
- 7488-55-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $180.00/kg2025-04-15
- CAS:
- 7488-55-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton