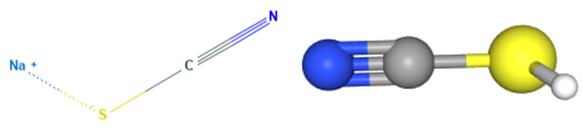
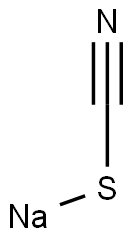Sodium Thiocyanate: A Comprehensive Guide for Chemical Industry Professionals
Sodium thiocyanate is a chemical raw material used as a solvent for spinning acrylic fibers, a chemical analysis reagent, a color film developer, a defoliant for certain plants, an airport road herbicide, etc. It is also used in pharmaceuticals, printing and dyeing, rubber processing, black nickel plating, and the manufacture of artificial mustard oil. It is often used in various industries such as medicine and printing and dyeing. It also has a certain antiseptic function. Adding a certain amount of sodium thiocyanate to milk can achieve the purpose of antibacterial, freshness preservation, and extending the shelf life of milk. In addition, sodium thiocyanate is also part of the animal body's defense system and participates in the body's life activities. Therefore, a certain amount of sodium thiocyanate naturally exists in raw milk.

Figure 1 Characteristics of Sodium thiocyanate
Safety risks of sodium thiocyanate
Sodium thiocyanate has a certain toxic effect on the human body. A small amount of intake is extremely harmful to the human body, and symptoms such as nausea, vomiting, and tremor may occur. It can inhibit thyroid function and prolong women's menstrual period. Excessive intake can cause acute toxicity, mainly manifested as nausea, vomiting, coma, organ failure and other symptoms.
Sodium thiocyanate limit value
In 2008, the former Ministry of Health of my country announced the "List of Non-food Substances and Food Additives that May Be Illegally Added to Food (First Batch)", which clearly stipulates that sodium thiocyanate is prohibited in milk and dairy products. Due to the background value in dairy products, in order to prevent potential risks, the State Administration for Market Regulation has set the risk monitoring reference value of sodium thiocyanate in liquid dairy products at 10mg/kg.
How to avoid risks
1. Consumers are advised to purchase milk through regular channels such as large supermarkets, and pay attention to the production date, shelf life, manufacturer and other information on the label.
2. Beware of milk with a long shelf life. Sodium thiocyanate may be added to milk and dairy products to prevent improper storage. For some milk and dairy products with a long shelf life, pay special attention.
3. After purchasing milk and other dairy products, they should be stored correctly according to the storage method on the label. If the packaging is bulging or damaged, it is not suitable for drinking.
4. Buy big-brand dairy products with guaranteed quality and pay attention to the blacklist exposed by the quality supervision department in a timely manner.
You may like
Related articles And Qustion
Lastest Price from Sodium thiocyanate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 540-72-7
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 540-72-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt