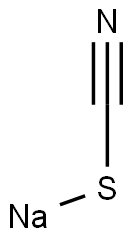Sodium thiocyanate-Application
Sodium thiocyanate(sometimes called sodium sulphocyanide) is an organic sodium salt which is the monosodium salt of thiocyanic acid. Sodium thiocyanate contains a thiocyanate. Sodium thiocyanate is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, Sodium thiocyanate is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals. Sodium thiocyanate, a white rhombic system crystal, is soluble in water, ethanol, acetone; Relative density is 1.735. Melts at approx. 287℃. Decomposes on heating and under influence of light producing toxic fumes of sulfur oxides,nitrogen oxides and cyanides. Reacts violently with acids,strong bases and strong oxidants[1,2].
Thiocyanate salts are typically prepared by the reaction of cyanide with elemental sulfur: 8 NaCN + S8 → 8 NaSCN
Sodium thiocyanate crystallizes in an orthorhombic cell. Each Na+ center is surrounded by three sulfur and three nitrogen ligands provided by the triatomic thiocyanate anion. Sodium thiocyanate is commonly used in the laboratory as a test for the presence of Fe3+ ions[3].
Sodium thiocyanate solution (56% or less) is an odorless clear to pale yellow liquid.
Sodium thiocyanate is employed to convert alkyl halides into the corresponding alkylthiocyanates. Closely related reagents include ammonium thiocyanate and potassium thiocyanate, which has twice the solubility in water. Silver thiocyanate may be used as well; the precipitation of insoluble silver halides help simplify workup. Treatment of isopropyl bromide with sodium thiocyanate in a hot ethanolic solution affords isopropyl thiocyanate. Protonation of sodium thiocyanate affords isothiocyanic acid, S=C=NH (pKa =-1.28). This species is generated in situ from sodium thiocyanate; Sodium thiocyanate adds to organic amines to afford derivatives of thiourea[4,5].
Sodium thiocyanate is a valuable chemical raw material and has important uses in various sectors of the national economy. Sodium thiocyanate is mainly used as a solvent for spinning acrylic fibers, chemical analysis reagents, color film film rinses, certain plant defoliants, and airport road herbicides. Sodium thiocyanate is also used in pharmaceuticals, printing and dyeing, rubber processing, black nickel plating, and artificial mustard. Oil, etc. The main industrial production processes are: separation of sodium thiocyanate from waste liquid in coke oven gas, synthesis of sodium thiocyanate with sodium cyanide and sulfur, and production of sodium thiocyanate by metathesis reaction of ammonium thiocyanate and sodium hydroxide. The synthesis of sodium thiocyanate requires high purity HCN as raw material, which is costly and harsh.
References
[1] Schwan A L. Sodium Thiocyanate[M]// e-EROS Encyclopedia of Reagents for Organic Synthesis. 2001.
[2] P. H. van Rooyen, J. C. A. Boeyens. Sodium thiocyanate[J]. Acta Crystallographica, 1975, 31(12):2933-2934.
[3] Cardenas R. Thermal properties of poly(ethylene oxide) complexed with sodium thiocyanate and potassium thiocyanate[J]. 2002, 20(12):231-236.
[4] Berchiesi G, Vitali G, Passamonti P. Viscoelastic relaxation in the acetamide + sodium thiocyanate binary system[J]. 1983, 79(8):1257-1263.
[5] Jian Wu, Zhiquan Shen. Ionic Conductivity of Ethylene Oxide and Propylene Oxide Copolymer Complexes with Sodium Thiocyanate[J]. Polymer Journal, 1990, 22(3):283-286.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium thiocyanate manufacturers

US $10.00/KG2026-01-05
- CAS:
- 540-72-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/KG2025-04-21
- CAS:
- 540-72-7
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month