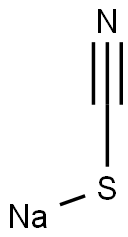Understanding the Diverse Applications and Potential Hazards of Sodium Thiocyanate
General Description
Sodium thiocyanate is a white crystalline powder with a molar mass of 81.07 g/mol and a melting point of approximately 300°C. It is highly soluble in water, ethanol, and acetone. This inorganic compound has various industrial applications, including as a solvent for spinning polyacrylonitrile fibers, a color film developer, a defoliant for certain plants, and a herbicide for airport runways. It is also used in the pharmaceutical, dyeing, rubber processing, black nickel plating, and pesticide industries. However, sodium thiocyanate poses potential health risks and environmental hazards. It can cause irritation, sensitization, and serious damage to human health and aquatic life. Proper safety measures should be taken when handling this chemical to minimize potential harm.

Figure 1. Sodium thiocyanate
Physical and Chemical Properties
Sodium thiocyanate has the chemical formula NaSCN and a molar mass of 81.07 g/mol. It is a white crystalline powder that readily absorbs moisture from the air, forming hygroscopic crystals or a white powder. The compound has a relatively high density of 1.7 g/cm³ and a melting point of approximately 572°F (300°C). It exhibits solubility in water, with a solubility of 139 g/100ml at 21°C, and it is also soluble in ethanol and acetone. In terms of its molecular properties, sodium thiocyanate has a molecular weight of 81.07 g/mol, and it contains two hydrogen bond acceptors. It does not function as a hydrogen bond donor and has no rotatable bonds. Additionally, the compound demonstrates a topological polar surface area of 24.8Ų. From an experimental standpoint, it is an odorless solid that sinks and mixes with water. Furthermore, it falls into the category of inorganic compounds within the class of metals. Lastly, it decomposes at a temperature of 368°C. These properties make sodium thiocyanate a significant compound with various potential applications in different fields. 1
Versatile Applications in Various Industries
Sodium thiocyanate, also known as sodium sulphocyanide, is a versatile inorganic compound with numerous industrial applications. It can be used as a solvent for spinning polyacrylonitrile fibers, as a color film developer, as a defoliant for certain plants, and as a herbicide for airport runways. In addition to these uses, it is also utilized in the pharmaceutical, dyeing, rubber processing, black nickel plating, color film, and pesticide industries. It is even employed as a mold inhibitor and preservative in some foods when used in combination with hydrogen peroxide. Sodium thiocyanate is also an important analytical reagent, particularly for the determination of niobium in steel and the measurement of silver, copper, and iron. Organic thiocyanates can also be produced using sodium thiocyanate. Overall, sodium thiocyanate has a broad range of practical applications due to its unique chemical properties. Its versatility and effectiveness in various fields make it an essential component in many industrial processes. 2
Potential hazards
Sodium thiocyanate poses potential hazards to human health and the environment. It is harmful if swallowed, inhaled, or in contact with the skin. Inhalation of its dust can cause irritation of the nose and throat, while ingestion of large doses can lead to vomiting, convulsions, and even death within 10-48 hours. Chronic exposure may result in flu-like symptoms, skin rashes, weakness, fatigue, vertigo, nausea, vomiting, diarrhea, and confusion. Contact with the eyes can cause irritation and serious damage, while prolonged skin exposure may result in various skin eruptions, dizziness, cramps, and mild to severe nervous system disturbances. Sodium thiocyanate has also been found to cause sensitization in humans and guinea pigs. Furthermore, it is harmful to aquatic life and can have long-lasting effects. It should be handled with caution to avoid environmental contamination. In summary, sodium thiocyanate presents multiple health risks and environmental hazards. Proper safety measures, including personal protective equipment and appropriate handling and storage practices, should be implemented when working with this chemical to minimize potential harm. 3
Reference
1. Sodium thiocyanate. National Center for Biotechnology Information, 2023, PubChem Compound Summary for CID 516871.
2. Zhong L, Chen G, Yang T, Gu J, Ma C, Li L, Wu Y, Zhu C, Gao H, Yang Z, Hu A, Xu J, Qiu X, Shen J, Huang A. Al2O3@Ag composite structure as SERS substrate for sensitive detection of sodium thiocyanate. Anal Sci. 2023 Apr;39(4):557-564.
3. Sodium thiocyanate. Haz-Map, Information on Hazardous Chemicals and Occupational Diseases, 9534.
Related articles And Qustion
See also
Lastest Price from Sodium thiocyanate manufacturers

US $0.00/KG2025-04-21
- CAS:
- 540-72-7
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $10.00/KG2025-04-21
- CAS:
- 540-72-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt