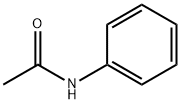
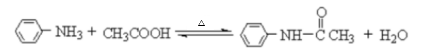
Polarity of Acetanilide
Polarity of Acetanilide
Acetanilide has several polar bonds, therefore, acetanilide is a polar molecule. Water is also a polar molecule, so acetanilide is moderately soluble in boiling water, but much less soluble in room temperature and cold water. Aniline is a potential impurity and is much more soluble in room temperature water. Therefore, first prepare a saturated solution of acetanilide in boiling water. Acetanilide is soluble in ethanol at room temperature. Therefore we cannot use ethanol as a solvent for recrystallisation of acetanilide. However, it is soluble in water when heated.
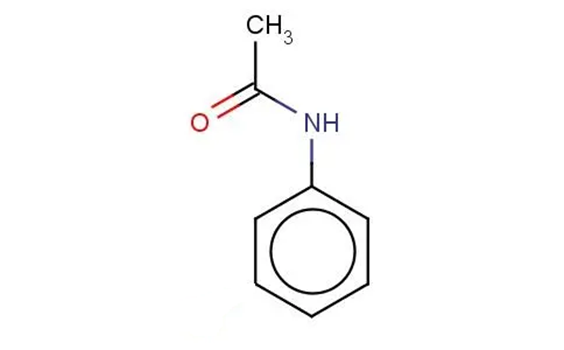
Why is acetanilide insoluble in cold water?
From the structure of acetanilide we can see that it has a large non-polar benzene ring, which makes it insoluble in water at low temperatures. However, the compound also has an acyl group which is polar. This will make the compound more soluble in water when heated.
Based on the structure of acetanilide, water is a good solvent for its recrystallisation. This is because recrystallisation is a technique that can be used to purify crude organic products. It involves dissolving the compound in a hot solvent and recrystallising it slowly as the solution cools. Good recrystallisation solvents must usually dissolve compounds at high temperatures, but not at lower temperatures.
Related articles And Qustion
See also
Lastest Price from Acetanilide manufacturers

US $10.00/kg2025-04-21
- CAS:
- 103-84-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $9.50/KG2024-10-11
- CAS:
- 103-84-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100ton