N,N-Dimethylformamide: Polarity and Application
Polarity of N,N-Dimethylformamide
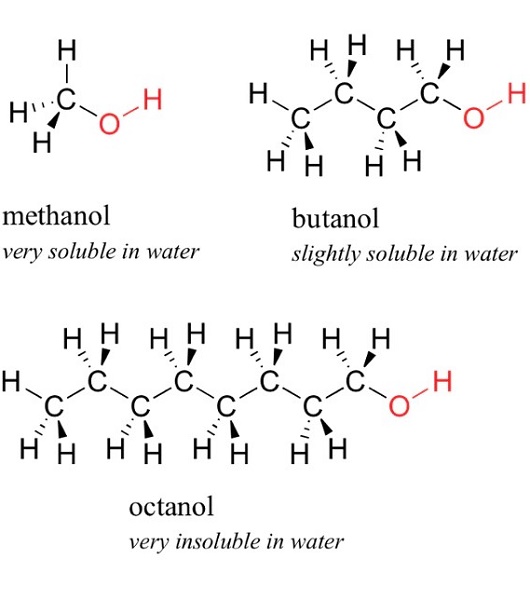
N,N-Dimethylformamide(DMF) is a colourless, odourless liquid solvent. It is miscible with water and many organic liquids. It has a polarity index of 6.4, a dipole moment of 3.86, and a dielectric constant of 37. It is known as a "universal solvent" and has been used in many commercial applications. It is similar in polarity to DMSO, but DMSO (dielectric constant = 49) is more polar solvent than DMF, (dielectric constant = 37). It is a polar non-boiling solvent because it has no OH or NH groups. Polar C=O and C-N bonds make the molecule polar. There are no O-H or N-H bonds, so the molecule does not boil.

Differences between DMF and DMSO solvents
DMF and DMSO are both good non-corrosive solvents. However, based on their dielectric constants, DMSO is more polar than DMF. Therefore, depending on the nature of the reaction conditions, you can use either of these two solvents. Both are equally polar, non-reactive solvents, but DMSO is safer than DMF.
Removal of N,N-Dimethylformamide
Removal of N,N-Dimethylformamide in polar solvents is carried out as follows: In the case of hazardous polar compounds, dilute with a large amount of water before extracting with a non-polar solvent. Then wash the organic layer thoroughly with water. Rule of thumb: For 5 mL of DMF or DMSO, use 5 X 10 mL of water in the aqueous wash. This will remove all of the DMF or DMSO.
Related articles And Qustion
See also
Lastest Price from N,N-Dimethylformamide manufacturers

US $0.00/kg2025-06-09
- CAS:
- Min. Order:
- 170kg
- Purity:
- 99%
- Supply Ability:
- 20 MT

US $0.00/kg2025-06-05
- CAS:
- Min. Order:
- 230kg
- Purity:
- 99%
- Supply Ability:
- 20 ton